State-dependent and timing-dependent bidirectional associative plasticity in the human SMA-M1 network
- PMID: 22031883
- PMCID: PMC6703519
- DOI: 10.1523/JNEUROSCI.2271-11.2011
State-dependent and timing-dependent bidirectional associative plasticity in the human SMA-M1 network
Abstract
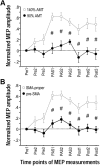
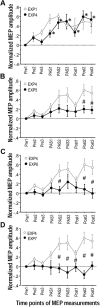
The supplementary motor area (SMA-proper) plays a key role in the preparation and execution of voluntary movements. Anatomically, SMA-proper is densely reciprocally connected to primary motor cortex (M1), but neuronal coordination within the SMA-M1 network and its modification by external perturbation are not well understood. Here we modulated the SMA-M1 network using MR-navigated multicoil associative transcranial magnetic stimulation in healthy subjects. Changes in corticospinal excitability were assessed by recording motor evoked potential (MEP) amplitude bilaterally in a hand muscle. We found timing-dependent bidirectional Hebbian-like MEP changes during and for at least 30 min after paired associative SMA-M1 stimulation. MEP amplitude increased if SMA stimulation preceded M1 stimulation by 6 ms, but decreased if SMA stimulation lagged M1 stimulation by 15 ms. This associative plasticity in the SMA-M1 network was highly topographically specific because paired associative stimulation of pre-SMA and M1 did not result in any significant MEP change. Furthermore, associative plasticity in the SMA-M1 network was strongly state-dependent because it required priming by near-simultaneous M1 stimulation to occur. We conclude that timing-dependent bidirectional associative plasticity is demonstrated for the first time at the systems level of a human corticocortical neuronal network. The properties of this form of plasticity are fully compatible with spike-timing-dependent plasticity as defined at the cellular level. The necessity of priming may reflect the strong interhemispheric connectivity of the SMA-M1 network. Findings are relevant for better understanding reorganization and potentially therapeutic modification of neuronal coordination in the SMA-M1 network after cerebral lesions such as stroke.
Figures


References
-
- Bestmann S, Baudewig J, Siebner HR, Rothwell JC, Frahm J. Subthreshold high-frequency TMS of human primary motor cortex modulates interconnected frontal motor areas as detected by interleaved fMRI-TMS. Neuroimage. 2003;20:1685–1696. - PubMed
-
- Bi G, Poo M. Synaptic modification by correlated activity: Hebb's postulate revisited. Annu Rev Neurosci. 2001;24:139–166. - PubMed
-
- Caporale N, Dan Y. Spike timing-dependent plasticity: a Hebbian learning rule. Annu Rev Neurosci. 2008;31:25–46. - PubMed
-
- Cincotta M, Borgheresi A, Jung P, Balestrieri F, Giovannelli F, Zaccara G, Ziemann U. Physical interactions between induced electrical fields can have substantial effects on neuronal excitation during simultaneous TMS of two brain areas. Clin Neurophysiol. 2005;116:1733–1742. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
