Saccharomyces cerevisiae chitin biosynthesis activation by N-acetylchitooses depends on size and structure of chito-oligosaccharides
- PMID: 22032207
- PMCID: PMC3221556
- DOI: 10.1186/1756-0500-4-454
Saccharomyces cerevisiae chitin biosynthesis activation by N-acetylchitooses depends on size and structure of chito-oligosaccharides
Abstract
Background: To explore chitin synthesis initiation, the effect of addition of exogenous oligosaccharides on in vitro chitin synthesis was studied. Oligosaccharides of various natures and lengths were added to a chitin synthase assay performed on a Saccharomyces cerevisiae membrane fraction.
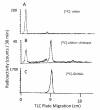
Findings: N-acetylchito-tetra, -penta and -octaoses resulted in 11 to 25% [14C]-GlcNAc incorporation into [14C]-chitin, corresponding to an increase in the initial velocity. The activation appeared specific to N-acetylchitooses as it was not observed with oligosaccharides in other series, such as beta-(1,4), beta-(1,3) or alpha-(1,6) glucooligosaccharides.
Conclusions: The effect induced by the N-acetylchitooses was a saturable phenomenon and did not interfere with free GlcNAc and trypsin which are two known activators of yeast chitin synthase activity in vitro. The magnitude of the activation was dependent on both oligosaccharide concentration and oligosaccharide size.
Figures




References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

