Feasibility and acceptability of a decision aid designed for people facing advanced or terminal illness: a pilot randomized trial
- PMID: 22032553
- PMCID: PMC5060696
- DOI: 10.1111/j.1369-7625.2011.00732.x
Feasibility and acceptability of a decision aid designed for people facing advanced or terminal illness: a pilot randomized trial
Abstract
Background: Patients nearing the end of their lives face an array of difficult decisions.
Objective: This study was designed to assess the feasibility and acceptability of a decision aid (DA) designed for patients facing advanced or terminal illness.
Design: We conducted a pilot randomized clinical trial of Health Dialog's Looking Ahead: choices for medical care when you're seriously ill DA (booklet and DVD) applied to patients on a hospital-based palliative care (PC) service.
Setting: University of Colorado Hospital - December 2009 and May 2010.
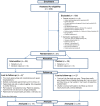
Participants: All adult, English-speaking patients or their decision makers were potentially eligible. Patients were not approached if they were in isolation, did not speak English or if any provider felt that they were not appropriate because of issues such as family conflict or actively dying.
Intervention: All participants received a standard PC consultation. Participants in the intervention arm also received a copy of the DA. Measurements Primary outcomes included decision conflict and knowledge. Participants in the intervention arm also completed an acceptability questionnaire and qualitative exit interviews.
Results: Of the 239 patients or decision makers, 51(21%) enrolled in the trial. The DA had no significant effect on decision conflict or knowledge. Exit interviews indicated it was acceptable and empowering, although they wished they had access to the DA earlier.
Conclusions: While the DA was acceptable, feasibility was limited by late-life illness challenges. Future trials of this DA should be performed on patients earlier in their illness trajectory and should include additional outcome measures such as self-efficacy and confidence.
Keywords: decision aid; end-of-life; palliative care; randomized trial.
© 2011 John Wiley & Sons Ltd.
Figures
References
-
- Singer PA, Martin DK, Kelner M. Quality end‐of‐life care. The Journal of the American Medical Association, 1999; 281: 163–168. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical