Epilepsy and the consciousness system: transient vegetative state?
- PMID: 22032662
- PMCID: PMC3432285
- DOI: 10.1016/j.ncl.2011.07.014
Epilepsy and the consciousness system: transient vegetative state?
Abstract
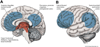
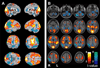
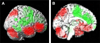
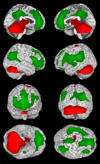
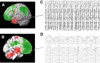
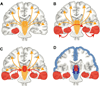
Recent advances have shown much in common between epilepsy and other disorders of consciousness. Behavior in epileptic seizures often resembles a transient vegetative or minimally conscious state. These disorders all converge on the "consciousness system" -the bilateral medial and lateral fronto-parietal association cortex and subcortical arousal systems. Epileptic unconsciousness has enormous clinical significance leading to accidental injuries, decreased work and school productivity, and social stigmatization. Ongoing research to better understand the mechanisms of impaired consciousness in epilepsy, including neuroimaging studies and fundamental animal models, will hopefully soon enable treatment trails to reduce epileptic unconsciousness and improve patient quality of life.
Copyright © 2011. Published by Elsevier Inc.
Figures

References
-
- Plum F, Posner JB. The diagnosis of stupor and coma. 3rd edition. Philadelphia: Davis; 1982.
-
- Blumenfeld H. Neuroanatomy through clinical cases. Sunderland (MA): Sinauer Assoc. Publ., Inc; 2002.
-
- Blumenfeld H. Epilepsy and consciousness. Chapter 2. In: Laureys S, editor. The neurology of consciousness: cognitive neuroscience and neuropathology. New York: G Tononi Academic Press; 2009. pp. 15–30.
-
- Blumenfeld H. Neuroanatomy through clinical cases. 2nd edition. Sunderland (MA): Sinauer Assoc Publ Co; 2010.
-
- Steriade M, Jones EG, McCormick DA, editors. Thalamus. Amsterdam: Elsevier Science; 1997.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

