Cardiovascular outcomes in the AFFIRM Trial (Atrial Fibrillation Follow-Up Investigation of Rhythm Management). An assessment of individual antiarrhythmic drug therapies compared with rate control with propensity score-matched analyses
- PMID: 22032709
- PMCID: PMC3290399
- DOI: 10.1016/j.jacc.2011.07.036
Cardiovascular outcomes in the AFFIRM Trial (Atrial Fibrillation Follow-Up Investigation of Rhythm Management). An assessment of individual antiarrhythmic drug therapies compared with rate control with propensity score-matched analyses
Abstract
Objectives: The impact of individual antiarrhythmic drugs (AADs) on mortality and hospital stay in atrial fibrillation (AF) was evaluated.
Background: Cardiovascular (CV) outcomes in AF patients receiving pharmacologic rhythm control therapy have not been compared with rate control therapy on the basis of AAD selection.
Methods: We compared CV outcomes in the AFFIRM (Atrial Fibrillation Follow-Up Investigation of Rhythm Management) trial in subgroups defined by the initial AAD selected with propensity score matched subgroups from the rate arm (Rate).
Results: Seven hundred twenty-nine amiodarone patients, 606 sotalol patients, and 268 Class 1C patients were matched. The composite outcome of mortality or cardiovascular hospital stays (CVH) showed better outcomes with Rate compared with amiodarone (hazard ratio [HR]: 1.18, 95% confidence interval [CI]: 1.03 to 1.36, p = 0.02), sotalol (HR: 1.32, 95% CI: 1.13 to 1.54, p < 0.001), and Class 1C (HR: 1.22, 95% CI: 0.97 to 1.56, p = 0.10). There was a nonsignificant increase in mortality with amiodarone (HR: 1.20, 95% CI: 0.94 to 1.53, p = 0.15) with the risk of non-CV death being significantly higher with amiodarone versus Rate (HR: 1.11, 95% CI: 1.01 to 1.24, p = 0.04). First CVH event rates at 3 years were 47% for amiodarone, 50% for sotalol, and 44% for Class 1C versus 40%, 40%, and 36%, respectively, for Rate (amiodarone HR: 1.20, 95% CI: 1.03 to 1.40, p = 0.02, sotalol HR: 1.364, 95% CI: 1.16 to 1.611, p < 0.001, Class 1C HR: 1.24, 95% CI: 0.96 to 1.60, p = 0.09). Time to CVH with intensive care unit stay or death was shorter with amiodarone (HR: 1.22, 95% CI: 1.02 to 1.46, p = 0.03).
Conclusions: In AFFIRM, composite mortality and CVH outcomes differed for Rate and AADs due to differences in CVH; CVH event rates during follow-up were high for all cohorts, but they were higher for all groups on AADs. Death, intensive care unit hospital stay, and non-CV death were more frequent with amiodarone.
Trial registration: ClinicalTrials.gov NCT00000556.
Copyright © 2011 American College of Cardiology Foundation. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Drs Reynolds and Freemantle are consultants for sanofi Aventis. Ms. Slee, Dr. Y. Rosenberg, Ms. S. Grant, Ms. E. Thomas, and Mr. S. Rathod have no conflicts of interest.
Figures

Hazard ratios and 95% confidence intervals (HR=Rhythm drug/Rate).
Propensity score matched Rate and amiodarone subgroups
Propensity score matched Rate and sotalol subgroups
Propensity score matched Rate and class1C subgroups.
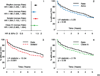
Hazard ratios and 95% confidence intervals (HR=Rhythm drug/Rate).
Propensity score matched Rate and amiodarone subgroups
Propensity score matched Rate and sotalol subgroups
Propensity score matched Rate and class 1C subgroups.
Hazard ratios and 95% confidence intervals (HR=Rhythm drug/Rate).
Propensity score matched Rate and amiodarone subgroups
Propensity score matched Rate and sotalol subgroups
Propensity score matched Rate and class 1C subgroups.

Hazard ratios and 95% confidence intervals (HR=Rhythm drug/Rate).
Propensity score matched Rate and amiodarone subgroups
Propensity score matched Rate and sotalol subgroups
Propensity score matched Rate and class 1C subgroups.
Hazard ratios and 95% confidence intervals (HR=Rhythm drug/Rate).
Propensity score matched Rate and amiodarone subgroups
Propensity score matched Rate and sotalol subgroups
Propensity score matched Rate and class 1C subgroups.
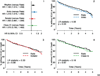
Hazard ratios and 95% confidence intervals (HR=Rhythm drug/Rate).
Propensity score matched Rate and amiodarone subgroups
Propensity score matched Rate and sotalol subgroups
Propensity score matched Rate and c 1C subgroups.
Hazard ratios and 95% confidence intervals (HR=Rhythm drug/Rate).
Propensity score matched Rate and amiodarone subgroups
Propensity score matched Rate and sotalol subgroups
Propensity score matched Rate and class1C subgroups.

Hazard ratios and 95% confidence intervals (HR=Rhythm drug/Rate).
Propensity score matched Rate and amiodarone subgroups
Propensity score matched Rate and sotalol subgroups
Propensity score matched Rate and c 1C subgroups.
Hazard ratios and 95% confidence intervals (HR=Rhythm drug/Rate).
Propensity score matched Rate and amiodarone subgroups
Propensity score matched Rate and sotalol subgroups
Propensity score matched Rate and class1C subgroups.
Comment in
-
Rhythm control for atrial fibrillation favorable outcomes or futile endeavor?J Am Coll Cardiol. 2011 Nov 1;58(19):1986-8. doi: 10.1016/j.jacc.2011.07.037. J Am Coll Cardiol. 2011. PMID: 22032710 No abstract available.
-
Antiarrhythmic drug therapy in 2012: time to finally open our eyes!J Am Coll Cardiol. 2012 Mar 13;59(11):1039-40; author reply 1040-1. doi: 10.1016/j.jacc.2011.11.041. J Am Coll Cardiol. 2012. PMID: 22402080 No abstract available.
References
-
- Benjamin EJ, Wolf PA, D'Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998 Sep 8;98(10):946–952. - PubMed
-
- AF Hospitalisations in the USA. 2009 June; www.nhlbi.nih.gov/resources/docs/cht-book.htm.
-
- The Planning and Steering Committees of the AFFIRM Study for the NHLBI AFFIRM Investigators. Atrial Fibrillation Follow-up Investigation of Rhythm Management -- the AFFIRM study design. Am J Cardiol. 1997;79:1198–1202. - PubMed
-
- The AFFIRM Investigators. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347:1825–1833. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

