Energy transfer in "parasitic" cancer metabolism: mitochondria are the powerhouse and Achilles' heel of tumor cells
- PMID: 22033146
- PMCID: PMC3272257
- DOI: 10.4161/cc.10.24.18487
Energy transfer in "parasitic" cancer metabolism: mitochondria are the powerhouse and Achilles' heel of tumor cells
Abstract
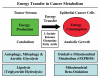
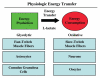
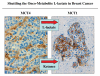
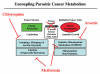
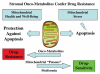
It is now widely recognized that the tumor microenvironment promotes cancer cell growth and metastasis via changes in cytokine secretion and extracellular matrix remodeling. However, the role of tumor stromal cells in providing energy for epithelial cancer cell growth is a newly emerging paradigm. For example, we and others have recently proposed that tumor growth and metastasis is related to an energy imbalance. Host cells produce energy-rich nutrients via catabolism (through autophagy, mitophagy, and aerobic glycolysis), which are then transferred to cancer cells to fuel anabolic tumor growth. Stromal cell-derived L-lactate is taken up by cancer cells and is used for mitochondrial oxidative phosphorylation (OXPHOS) to produce ATP efficiently. However, "parasitic" energy transfer may be a more generalized mechanism in cancer biology than previously appreciated. Two recent papers in Science and Nature Medicine now show that lipolysis in host tissues also fuels tumor growth. These studies demonstrate that free fatty acids produced by host cell lipolysis are re-used via beta-oxidation (beta-OX) in cancer cell mitochondria. Thus, stromal catabolites (such as lactate, ketones, glutamine and free fatty acids) promote tumor growth by acting as high-energy onco-metabolites. As such, host catabolism, via autophagy, mitophagy and lipolysis, may explain the pathogenesis of cancer-associated cachexia and provides exciting new druggable targets for novel therapeutic interventions. Taken together, these findings also suggest that tumor cells promote their own growth and survival by behaving as a "parasitic organism." Hence, we propose the term "Parasitic Cancer Metabolism" to describe this type of metabolic coupling in tumors. Targeting tumor cell mitochondria (OXPHOS and beta-OX) would effectively uncouple tumor cells from their hosts, leading to their acute starvation. In this context, we discuss new evidence that high-energy onco-metabolites (produced by the stroma) can confer drug resistance. Importantly, this metabolic chemo-resistance is reversed by blocking OXPHOS in cancer cell mitochondria with drugs like Metformin, a mitochondrial "poison." In summary, parasitic cancer metabolism is achieved architecturally by dividing tumor tissue into at least two well-defined opposing "metabolic compartments:" catabolic and anabolic.
Figures




References
-
- Hart IR, Fidler IJ. Role of organ selectivity in the determination of metastatic patterns of B16 melanoma. Cancer Res. 1980;40:2281–2287. - PubMed
Publication types
MeSH terms
Grants and funding
- R01 CA075503/CA/NCI NIH HHS/United States
- R01 CA098779/CA/NCI NIH HHS/United States
- R01-CA-120876/CA/NCI NIH HHS/United States
- R01 CA120876/CA/NCI NIH HHS/United States
- R01-CA-70896/CA/NCI NIH HHS/United States
- R01-CA-098779/CA/NCI NIH HHS/United States
- R01-CA-86072/CA/NCI NIH HHS/United States
- R01-CA-080250/CA/NCI NIH HHS/United States
- R01 CA070896/CA/NCI NIH HHS/United States
- R01 CA107382/CA/NCI NIH HHS/United States
- P30-CA-56036/CA/NCI NIH HHS/United States
- R01-CA-107382/CA/NCI NIH HHS/United States
- AI-76248/AI/NIAID NIH HHS/United States
- R01-AR-055660/AR/NIAMS NIH HHS/United States
- R01 AR055660/AR/NIAMS NIH HHS/United States
- R01-CA-75503/CA/NCI NIH HHS/United States
- R01 CA080250/CA/NCI NIH HHS/United States
- R01 CA086072/CA/NCI NIH HHS/United States
- R01 AI076248/AI/NIAID NIH HHS/United States
- P30 CA056036/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
