HMGB1, an alarmin promoting HIV dissemination and latency in dendritic cells
- PMID: 22033335
- PMCID: PMC3252828
- DOI: 10.1038/cdd.2011.134
HMGB1, an alarmin promoting HIV dissemination and latency in dendritic cells
Abstract
Dendritic cells (DCs) initiate immune responses by transporting antigens and migrating to lymphoid tissues to initiate T-cell responses. DCs are located in the mucosal surfaces that are involved in human immunodeficiency virus (HIV) transmission and they are probably among the earliest targets of HIV-1 infection. DCs have an important role in viral transmission and dissemination, and HIV-1 has evolved different strategies to evade DC antiviral activity. High mobility group box 1 (HMGB1) is a DNA-binding nuclear protein that can act as an alarmin, a danger signal to alert the innate immune system for the initiation of host defense. It is the prototypic damage-associated molecular pattern molecule, and it can be secreted by innate cells, including DCs and natural killer (NK) cells. The fate of DCs is dependent on a cognate interaction with NK cells, which involves HMGB1 expressed at NK-DC synapse. HMGB1 is essential for DC maturation, migration to lymphoid tissues and functional type-1 polarization of naïve T cells. This review highlights the latest advances in our understanding of the impact of HIV on the interactions between HMGB1 and DCs, focusing on the mechanisms of HMGB1-dependent viral dissemination and persistence in DCs, and discussing the consequences on antiviral innate immunity, immune activation and HIV pathogenesis.
Figures




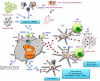
References
-
- Thomas JO, Travers AA. HMG1 and 2, and related ‘architectural' DNA-binding proteins. Trends Biochem Sci. 2001;26:167–174. - PubMed
-
- Stros M, Ozaki T, Bacikova A, Kageyama H, Nakagawara A. HMGB1 and HMGB2 cell-specifically down-regulate the p53-and p73-dependent sequence-specific transactivation from the human Bax gene promoter. J Biol Chem. 2002;277:7157–7164. - PubMed
-
- Stros M, Muselikova-Polanska E, Pospisilova S, Strauss F. High-affinity binding of tumor-suppressor protein p53 and HMGB1 to hemicatenated DNA loops. Biochemistry. 2004;43:7215–7225. - PubMed
Relevant references
-
- Wang HC, Bloom O, Zhang MH, Vishnubhakat JM, Ombrellino M, Che JT, et al. HMG-1 as a late mediator of endotoxin lethality in mice Science 1999285248–251.First demontration of the in vivo involvement and lethality of HMG-1 in a murine model of endotoxemia. - PubMed
-
- Yanai H, Ban T, Wang ZC, Choi MK, Kawamura T, Negishi H, et al. HMGB proteins function as universal sentinels for nucleic-acid-mediated innate immune responses Nature 2009462U99–U110.Elegant study showing that HMGB proteins function as nucleic-acid-sensing systems, their absence severely impairing nucleic-acid-mediated TLRs activation and induction of innate responses. - PubMed
-
- Scaffidi P, Misteli T, Bianchi ME.Release of chromatin protein HMGB1 by necrotic cells triggers inflammation Nature 2002418191–195.Cells undergoing necrosis release HMGB1 that signals the demise of a cell to its neighbours. In contrast, cells undergoing apoptosis are programmed to withhold this signal, thus not triggering inflammation. - PubMed
-
- Dumitriu IE, Bianchi ME, Bacci M, Manfredi AA, Rovere-Querini P.The secretion of HMGB1 is required for the migration of maturing dendritic cells J Leu Biol 20078184–91.The migratory functions of human DCs require the autocrine/paracrine release of HMGB1, which upregulates chemokine receptors and induces their response to chemokine-receptor ligands in a RAGE-dependent manner. - PubMed
-
- Semino C, Angelini G, Poggi A, Rubartelli A.NK/iDC interaction results in IL-18 secretion by DCs at the synaptic cleft followed by NK cell activation and release of the DC maturation factor HMGB1 Blood 2005106609–616.First demonstration of the essential role of HMGB1 in NK–DC crosstalk. IL-18 produced by DCs activates NK cells to release HMGB1, which induces DC maturation, thus favoring the onset of the adaptive immune response. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

