Dual modification of Alzheimer's disease PHF-tau protein by lysine methylation and ubiquitylation: a mass spectrometry approach
- PMID: 22033876
- PMCID: PMC3249157
- DOI: 10.1007/s00401-011-0893-0
Dual modification of Alzheimer's disease PHF-tau protein by lysine methylation and ubiquitylation: a mass spectrometry approach
Abstract
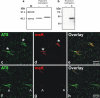
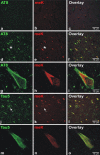
In sporadic Alzheimer's disease (AD), neurofibrillary lesion formation is preceded by extensive post-translational modification of the microtubule associated protein tau. To identify the modification signature associated with tau lesion formation at single amino acid resolution, immunopurified paired helical filaments were isolated from AD brain and subjected to nanoflow liquid chromatography-tandem mass spectrometry analysis. The resulting spectra identified monomethylation of lysine residues as a new tau modification. The methyl-lysine was distributed among seven residues located in the projection and microtubule binding repeat regions of tau protein, with one site, K254, being a substrate for a competing lysine modification, ubiquitylation. To characterize methyl lysine content in intact tissue, hippocampal sections prepared from post mortem late-stage AD cases were subjected to double-label confocal fluorescence microscopy using anti-tau and anti-methyl lysine antibodies. Anti-methyl lysine immunoreactivity colocalized with 78 ± 13% of neurofibrillary tangles in these specimens. Together these data provide the first evidence that tau in neurofibrillary lesions is post-translationally modified by lysine methylation.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

