AAV-mediated liver-directed gene therapy
- PMID: 22034029
- PMCID: PMC4118577
- DOI: 10.1007/978-1-61779-370-7_6
AAV-mediated liver-directed gene therapy
Abstract
The liver is directly or indirectly involved in many essential processes and is affected by numerous inherited diseases. Therefore, many inherited diseases could be effectively treated by targeting the liver using gene transfer approaches. The challenges associated with liver-directed gene therapy are efficient targeting of hepatocytes, stability of the vector genome, and persistent high level expression. Many of these obstacles can be overcome with adeno-associated viral (AAV) gene transfer vectors. The first AAV gene transfer -vector developed for in vivo use was based on the AAV2 serotype. AAV2 has a broad tropism and transduces many cell types, including hepatocytes, relatively efficiently in vivo. The capsid protein confers the serological profile and at least 12 primate AAV serotypes have already been characterized. Importantly, pseudotyping a recombinant AAV vector with different capsid proteins can dramatically alter the tropism. Both AAV8 and AAV9 have higher affinities for hepatocytes when compared to AAV2. In particular, AAV8 can transduce three- to fourfold more hepatocytes and deliver three- to fourfold more genomes per transduced cell when compared to AAV2. Depending on the dose, AAV8 can transduce up to 90-95% of hepatocytes in the mouse liver following intraportal vein injection. Interestingly, comparable levels of transduction can be achieved following intravenous injection. Direct intraparenchymal injection of an AAV vector also mediates relatively high level long term expression. Additional specificity can be conferred by using liver-specific promoters in conjunction with AAV8 capsid proteins. In addition to treating primary hepatocyte defects, immune reactions to transgene products can be minimized by circumventing the fixed tissue macrophages of the liver, Kupffer cells, and limiting expression to hepatocytes. The ability to target hepatocytes by virtue of the AAV serotype and the use of liver-specific promoters allows investigators to test novel therapeutic approaches and answer basic clinical and biological questions.
Figures
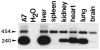

References
-
- Goldman L, Ausiello D, editors. Cecil Textbook of Medicine. 22. Saunders; Philadelphia, PA: 2004.
-
- Grimm D, Kern A, Rittner K, Kleinschmidt JA. Novel tools for production and purification of recombinant adeno-associated virus vectors. Hum Gene Ther. 1998;9:2745–60. - PubMed
-
- Zolotukhin S, Byrne BJ, Mason E, Zolotukhin I, Potter M, Chesnut K, Summerford C, Samulski RJ, Muzyzcka N. Recombinant adeno-associated virus purification using novel methods improves infectious titer and yield. Gene Ther. 1999;6:973–85. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

