Human breast cancer histoid: an in vitro 3-dimensional co-culture model that mimics breast cancer tissue
- PMID: 22034518
- PMCID: PMC3283087
- DOI: 10.1369/0022155411423680
Human breast cancer histoid: an in vitro 3-dimensional co-culture model that mimics breast cancer tissue
Abstract
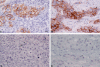
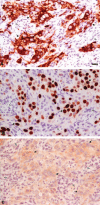
Progress in our understanding of heterotypic cellular interaction in the tumor microenvironment, which is recognized to play major roles in cancer progression, has been hampered due to unavailability of an appropriate in vitro co-culture model. The aim of this study was to generate an in vitro 3-dimensional human breast cancer model, which consists of cancer cells and fibroblasts. Breast cancer cells (UACC-893) and fibroblasts at various densities were co-cultured in a rotating suspension culture system to establish co-culture parameters. Subsequently, UACC-893, BT.20, or MDA.MB.453 were co-cultured with fibroblasts for 9 days. Co-cultures resulted in the generation of breast cancer histoid (BCH) with cancer cells showing the invasion of fibroblast spheroids, which were visualized by immunohistochemical (IHC) staining of sections (4 µm thick) of BCH. A reproducible quantitative expression of C-erbB.2 was detected in UACC-893 cancer cells in BCH sections by IHC staining and the Automated Cellular Imaging System. BCH sections also consistently exhibited qualitative expression of pancytokeratins, p53, Ki-67, or E-cadherin in cancer cells and that of vimentin or GSTPi in fibroblasts, fibronectin in the basement membrane and collagen IV in the extracellular matrix. The expression of the protein analytes and cellular architecture of BCH were markedly similar to those of breast cancer tissue.
Conflict of interest statement
The authors declared no potential conflicts of interest with respect to the authorship and publication of this article.
Figures



References
-
- Burstein HJ, Chen Y-H, Parker LM, et al. 2008. VEGF as a marker for outcome among advanced breast cancer patients receiving anti-VEGF therapy with bevacizumab and vinorelbine chemotherapy. Clin Cancer Res. 14:7871–7877 - PubMed
-
- Che ZM, Jung TH, Choi JH, et al. 2006. Collagen-based co-culture for invasive study on cancer cells-fibroblasts interaction. Biochem Biophys Res Commun. 346:268–275 - PubMed
-
- Chung LW, Baseman A, Assikis V, et al. 2005. Molecular insights into prostate cancer progression: the missing link of tumor microenvironment. J Urol. 173:10–20 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

