Proteome-wide post-translational modification statistics: frequency analysis and curation of the swiss-prot database
- PMID: 22034591
- PMCID: PMC3201773
- DOI: 10.1038/srep00090
Proteome-wide post-translational modification statistics: frequency analysis and curation of the swiss-prot database
Abstract
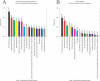
Post-translational modifications (PTMs) broadly contribute to the recent explosion of proteomic data and possess a complexity surpassing that of protein design. PTMs are the chemical modification of a protein after its translation, and have wide effects broadening its range of functionality. Based on previous estimates, it is widely believed that more than half of proteins are glycoproteins. Whereas mutations can only occur once per position, different forms of post-translational modifications may occur in tandem. With the number and abundances of modifications constantly being discovered, there is no method to readily assess their relative levels. Here we report the relative abundances of each PTM found experimentally and putatively, from high-quality, manually curated, proteome-wide data, and show that at best, less than one-fifth of proteins are glycosylated. We make available to the academic community a continuously updated resource (http://selene.princeton.edu/PTMCuration) containing the statistics so scientists can assess "how many" of each PTM exists.
Figures
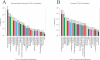

References
-
- Apweiler R., Hermjakob H. & Sharon N. On the frequency of protein glycosylation, as deduced from analysis of the SWISS-PROT database. Biochim. Biophys. Acta, Gen. Subj. 1473, 4–8 (1999). - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

