Protein trafficking dysfunctions: Role in the pathogenesis of pulmonary arterial hypertension
- PMID: 22034594
- PMCID: PMC3198637
- DOI: 10.4103/2045-8932.78097
Protein trafficking dysfunctions: Role in the pathogenesis of pulmonary arterial hypertension
Abstract
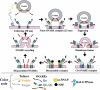
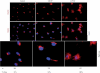
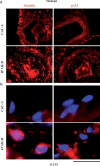
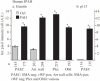
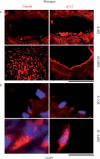
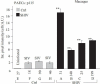
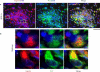
Earlier electron microscopic data had shown that a hallmark of the vascular remodeling in pulmonary arterial hypertension (PAH) in man and experimental models includes enlarged vacuolated endothelial and smooth muscle cells with increased endoplasmic reticulum and Golgi stacks in pulmonary arterial lesions. In cell culture and in vivo experiments in the monocrotaline model, we observed disruption of Golgi function and intracellular trafficking with trapping of diverse vesicle tethers, SNAREs and SNAPs in the Golgi membranes of enlarged pulmonary arterial endothelial cells (PAECs) and pulmonary arterial smooth muscle cells (PASMCs). Consequences included the loss of cell surface caveolin-1, hyperactivation of STAT3, mislocalization of eNOS with reduced cell surface/caveolar NO and hypo-S-nitrosylation of trafficking-relevant proteins. Similar Golgi tether, SNARE and SNAP dysfunctions were also observed in hypoxic PAECs in culture and in PAECs subjected to NO scavenging. Strikingly, a hypo-NO state promoted PAEC mitosis and cell proliferation. Golgi dysfunction was also observed in pulmonary vascular cells in idiopathic PAH (IPAH) in terms of a marked cytoplasmic dispersal and increased cellular content of the Golgi tethers, giantin and p115, in cells in the proliferative, obliterative and plexiform lesions in IPAH. The question of whether there might be a causal relationship between trafficking dysfunction and vasculopathies of PAH was approached by genetic means using HIV-nef, a protein that disrupts endocytic and trans-Golgi trafficking. Macaques infected with a chimeric simian immunodeficiency virus (SIV) containing the HIV-nef gene (SHIV-nef), but not the non-chimeric SIV virus containing the endogenous SIV-nef gene, displayed pulmonary arterial vasculopathies similar to those in human IPAH. Only macaques infected with chimeric SHIV-nef showed pulmonary vascular lesions containing cells with dramatic cytoplasmic dispersal and increase in giantin and p115. Specifically, it was the HIV-nef-positive cells that showed increased giantin. Elucidating how each of these changes fits into the multifactorial context of hypoxia, reduced NO bioavailability, mutations in BMPR II, modulation of disease penetrance and gender effects in disease occurrence in the pathogenesis of PAH is part of the road ahead.
Keywords: Golgi apparatus; Pulmonary vascular remodeling; intracellular organelles.
Conflict of interest statement
Figures





References
-
- Stenmark KR, Meyrick B, Galie N, Mooi WJ, McMurtry IF. Animal models of pulmonary arterial hypertension: The hope for etiological discovery and pharmacological cure. Am J Physiol Lung Cell Mol Physiol. 2009;297:L1013–32. - PubMed
-
- Mathew R, Huang J, Shah M, Patel K, Gewitz M, Sehgal PB. Disruption of endothelial-cell caveolin-1alpha/raft scaffolding during development of monocrotaline-induced pulmonary hypertension. Circulation. 2004;110:1499–506. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

