Multi-path quenchers: efficient quenching of common fluorophores
- PMID: 22034828
- PMCID: PMC3226780
- DOI: 10.1021/bc200424r
Multi-path quenchers: efficient quenching of common fluorophores
Abstract
Fluorescence quenching groups are widely employed in biological detection, sensing, and imaging. To date, a relatively small number of such groups are in common use. Perhaps the most commonly used quencher, dabcyl, has limited efficiency with a broad range of fluorophores. Here, we describe a molecular approach to improve the efficiency of quenchers by increasing their electronic complexity. Multi-Path Quenchers (MPQ) are designed to have multiple donor or acceptor groups in their structure, allowing for a multiplicity of conjugation pathways of varied length. This has the effect of broadening the absorption spectrum, which in turn can increase quenching efficiency and versatility. Six such MPQ derivatives are synthesized and tested for quenching efficiency in a DNA hybridization context. Duplexes placing quenchers and fluorophores within contact distance or beyond this distance are used to measure quenching via contact or FRET mechanisms. Results show that several of the quenchers are considerably more efficient than dabcyl at quenching a wider range of common fluorophores, and two quench fluorescein and TAMRA as well as or better than a Black Hole Quencher.
Figures
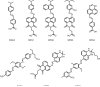
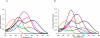
 ), MPQ2 (
), MPQ2 ( ), MPQ3 (
), MPQ3 ( ), MPQ4 (
), MPQ4 ( ), MPQ5 (
), MPQ5 ( ), MPQ6 (
), MPQ6 ( ), Dabcyl (
), Dabcyl ( ), BHQ2 (
), BHQ2 ( ).
).
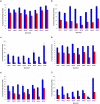
 ) and mixed mechanism (
) and mixed mechanism ( ) quenching with (a) AlexaFluor 350, (b) Fluorescein, (c) Cy 3, (d) TAMRA, (e) ATTO 590 and (f) Quasar 670.
) quenching with (a) AlexaFluor 350, (b) Fluorescein, (c) Cy 3, (d) TAMRA, (e) ATTO 590 and (f) Quasar 670.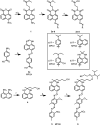
References
-
- Tyagi S, Kramer FR. Molecular beacons: probes that fluoresce upon hybridization. Nat. Biotechnol. 1996;14(3):303–308. - PubMed
-
- Nakayama S, Yan L, Sintim HO. Junction probes – sequence specific detection of nucleic acids via template enhanced hybridization processes. J. Am. Chem. Soc. 2008;130(38):12560–12561. - PubMed
-
- Li J, Wang F, Mamon H, Kulke MH, Harris L, Maher E, Wang L, Makrigiorgos GM. Antiprimer quenching-based real-time PCR and its application to the analysis of clinical cancer samples. Clinical Chem. 2006;52(4):624–633. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

