Early rehabilitation in sepsis: a prospective randomised controlled trial investigating functional and physiological outcomes The i-PERFORM Trial (Protocol Article)
- PMID: 22035174
- PMCID: PMC3306201
- DOI: 10.1186/1471-2253-11-21
Early rehabilitation in sepsis: a prospective randomised controlled trial investigating functional and physiological outcomes The i-PERFORM Trial (Protocol Article)
Abstract
Background: Patients with sepsis syndromes in comparison to general intensive care patients can have worse outcomes for physical function, quality of life and survival. Early intensive care rehabilitation can improve the outcome in general Intensive Care Unit (ICU) patients, however no investigations have specifically looked at patients with sepsis syndromes. The 'i-PERFORM Trial' will investigate if early targeted rehabilitation is both safe and effective in patients with sepsis syndromes admitted to ICU.
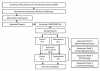
Methods/design: A single-centred blinded randomized controlled trial will be conducted in Brisbane, Australia. Participants (n = 252) will include those ≥ 18 years, mechanically ventilated for ≥ 48 hours and diagnosed with a sepsis syndrome. Participants will be randomised to an intervention arm which will undergo an early targeted rehabilitation program according to the level of arousal, strength and cardiovascular stability and a control group which will receive normal care.The primary outcome measures will be physical function tests on discharge from ICU (The Acute Care Index of Function and The Physical Function ICU Test). Health-related quality of life will be measured using the Short Form-36 and the psychological component will be tested using The Hospital Anxiety and Depression Scale. Secondary measures will include inflammatory biomarkers; Interleukin-6, Interleukin-10 and Tumour Necrosis Factor-α, peripheral blood mitochondrial DNA content and lactate, fat free muscle mass, tissue oxygenation and microcirculatory flow.
Discussion: The 'i-PERFORM Trial' will determine whether early rehabilitation for patients with sepsis is effective at improving patient outcomes with functional and physiological parameters reflecting long and short-term effects of early exercise and the safety in its application in critical illness.
Trial registration: Australia and New Zealand Clinical Trials Register (ANZCTR): ACTRN12610000808044.
Figures

Similar articles
-
Early physical rehabilitation in intensive care patients with sepsis syndromes: a pilot randomised controlled trial.Intensive Care Med. 2015 May;41(5):865-74. doi: 10.1007/s00134-015-3763-8. Epub 2015 Apr 8. Intensive Care Med. 2015. PMID: 25851383 Clinical Trial.
-
Therapeutic respiratory and functional rehabilitation protocol for intensive care unit patients affected by COVID-19: a structured summary of a study protocol for a randomised controlled trial.Trials. 2021 Apr 12;22(1):268. doi: 10.1186/s13063-021-05210-y. Trials. 2021. PMID: 33845878 Free PMC article.
-
Critical Care Cycling Study (CYCLIST) trial protocol: a randomised controlled trial of usual care plus additional in-bed cycling sessions versus usual care in the critically ill.BMJ Open. 2017 Oct 22;7(10):e017393. doi: 10.1136/bmjopen-2017-017393. BMJ Open. 2017. PMID: 29061618 Free PMC article. Clinical Trial.
-
Early rehabilitation in critical care (eRiCC): functional electrical stimulation with cycling protocol for a randomised controlled trial.BMJ Open. 2012 Sep 13;2(5):e001891. doi: 10.1136/bmjopen-2012-001891. Print 2012. BMJ Open. 2012. PMID: 22983782 Free PMC article.
-
Behavioural modification interventions for medically unexplained symptoms in primary care: systematic reviews and economic evaluation.Health Technol Assess. 2020 Sep;24(46):1-490. doi: 10.3310/hta24460. Health Technol Assess. 2020. PMID: 32975190 Free PMC article.
Cited by
-
IMPOSE (IMProving Outcomes after Sepsis)-the effect of a multidisciplinary follow-up service on health-related quality of life in patients postsepsis syndromes-a double-blinded randomised controlled trial: protocol.BMJ Open. 2014 May 26;4(5):e004966. doi: 10.1136/bmjopen-2014-004966. BMJ Open. 2014. PMID: 24861549 Free PMC article. Clinical Trial.
-
Assessment and predictors of physical functioning post-hospital discharge in survivors of critical illness.Ann Intensive Care. 2016 Dec;6(1):92. doi: 10.1186/s13613-016-0187-8. Epub 2016 Sep 20. Ann Intensive Care. 2016. PMID: 27646108 Free PMC article.
-
Clinical review: early patient mobilization in the ICU.Crit Care. 2013 Feb 28;17(1):207. doi: 10.1186/cc11820. Crit Care. 2013. PMID: 23672747 Free PMC article. Review.
-
Early physical rehabilitation in intensive care patients with sepsis syndromes: a pilot randomised controlled trial.Intensive Care Med. 2015 May;41(5):865-74. doi: 10.1007/s00134-015-3763-8. Epub 2015 Apr 8. Intensive Care Med. 2015. PMID: 25851383 Clinical Trial.
-
Mobilization in severe sepsis: an integrative review.J Hosp Med. 2015 Jan;10(1):54-9. doi: 10.1002/jhm.2281. J Hosp Med. 2015. PMID: 25393649 Free PMC article. Review.
References
-
- Brun-Buisson C, Doyon F, Carlet J, Dellamonica P, Gouin F, Lepoutre A, Mercier J, Offenstadt G, Régnier B. Incidence, risk factors, and outcome of severe sepsis and septic shock in adults. A multicentre prospective study in intensive care in adults. JAMA. 1995;274(12):968–74. doi: 10.1001/jama.274.12.968. - DOI - PubMed
-
- Heyland D, Hopman W, Coo H, Tranmer J, McColl M. Long term health related quality of life in survivors of sepsis. Short Form 36: a valid and reliable measure of health related quality of life. Crit Care Med. 2001;28:3599–3605. - PubMed
LinkOut - more resources
Full Text Sources

