Long-term effect of aspirin on cancer risk in carriers of hereditary colorectal cancer: an analysis from the CAPP2 randomised controlled trial
- PMID: 22036019
- PMCID: PMC3243929
- DOI: 10.1016/S0140-6736(11)61049-0
Long-term effect of aspirin on cancer risk in carriers of hereditary colorectal cancer: an analysis from the CAPP2 randomised controlled trial
Abstract
Background: Observational studies report reduced colorectal cancer in regular aspirin consumers. Randomised controlled trials have shown reduced risk of adenomas but none have employed prevention of colorectal cancer as a primary endpoint. The CAPP2 trial aimed to investigate the antineoplastic effects of aspirin and a resistant starch in carriers of Lynch syndrome, the major form of hereditary colorectal cancer; we now report long-term follow-up of participants randomly assigned to aspirin or placebo.
Methods: In the CAPP2 randomised trial, carriers of Lynch syndrome were randomly assigned in a two-by-two factorial design to 600 mg aspirin or aspirin placebo or 30 g resistant starch or starch placebo, for up to 4 years. Randomisation was in blocks of 16 with provision for optional single-agent randomisation and extended postintervention double-blind follow-up; participants and investigators were masked to treatment allocation. The primary endpoint was development of colorectal cancer. Analysis was by intention to treat and per protocol. This trial is registered, ISRCTN59521990.
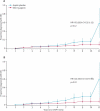
Results: 861 participants were randomly assigned to aspirin or aspirin placebo. At a mean follow-up of 55·7 months, 48 participants had developed 53 primary colorectal cancers (18 of 427 randomly assigned to aspirin, 30 of 434 to aspirin placebo). Intention-to-treat analysis of time to first colorectal cancer showed a hazard ratio (HR) of 0·63 (95% CI 0·35-1·13, p=0·12). Poisson regression taking account of multiple primary events gave an incidence rate ratio (IRR) of 0·56 (95% CI 0·32-0·99, p=0·05). For participants completing 2 years of intervention (258 aspirin, 250 aspirin placebo), per-protocol analysis yielded an HR of 0·41 (0·19-0·86, p=0·02) and an IRR of 0·37 (0·18-0·78, p=0·008). No data for adverse events were available postintervention; during the intervention, adverse events did not differ between aspirin and placebo groups.
Interpretation: 600 mg aspirin per day for a mean of 25 months substantially reduced cancer incidence after 55·7 months in carriers of hereditary colorectal cancer. Further studies are needed to establish the optimum dose and duration of aspirin treatment.
Funding: European Union; Cancer Research UK; Bayer Corporation; National Starch and Chemical Co; UK Medical Research Council; Newcastle Hospitals trustees; Cancer Council of Victoria Australia; THRIPP South Africa; The Finnish Cancer Foundation; SIAK Switzerland; Bayer Pharma.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures

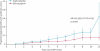
Comment in
-
Aspirin and colorectal cancer prevention in Lynch syndrome.Lancet. 2011 Dec 17;378(9809):2051-2. doi: 10.1016/S0140-6736(11)61216-6. Epub 2011 Oct 27. Lancet. 2011. PMID: 22036018 Free PMC article. No abstract available.
-
Disease prevention: Pain killer--cancer preventer.Nat Rev Clin Oncol. 2011 Nov 15;9(1):4. doi: 10.1038/nrclinonc.2011.175. Nat Rev Clin Oncol. 2011. PMID: 22083040 No abstract available.
-
Does aspirin really reduce the risk of colon cancer?Lancet. 2012 Apr 28;379(9826):1586-7; author reply 1587. doi: 10.1016/S0140-6736(12)60672-2. Epub 2012 Apr 26. Lancet. 2012. PMID: 22541573 No abstract available.
-
Does aspirin really reduce the risk of colon cancer?Lancet. 2012 Apr 28;379(9826):1586; author reply 1587. doi: 10.1016/S0140-6736(12)60671-0. Epub 2012 Apr 26. Lancet. 2012. PMID: 22541575 No abstract available.
References
-
- Vasen HFA, Watson P, Mecklin J-P, Lynch HT. New clinical criteria for hereditary nonpolyposis colorectal cancer (HNPCC, Lynch Syndrome) proposed by the International Collaborative Group on HNPCC. Gastroenterology. 1999;116:1453–1456. - PubMed
-
- Burn J, Bishop DT, Mecklin J-P. Effect of aspirin or resistant starch on colorectal neoplasia in the Lynch syndrome. N Engl J Med. 2008;359:2567–2578. - PubMed
-
- Giovannucci E, Egan KM, Hunter DJ. Aspirin and the risk of colorectal cancer in women. N Engl J Med. 1995;333:609–614. - PubMed

