Endothelial-derived angiocrine signals induce and sustain regenerative lung alveolarization
- PMID: 22036563
- PMCID: PMC3228268
- DOI: 10.1016/j.cell.2011.10.003
Endothelial-derived angiocrine signals induce and sustain regenerative lung alveolarization
Abstract
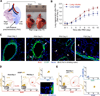
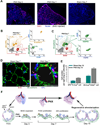
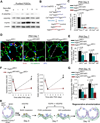
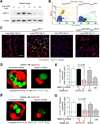
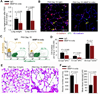
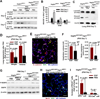
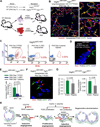
To identify pathways involved in adult lung regeneration, we employ a unilateral pneumonectomy (PNX) model that promotes regenerative alveolarization in the remaining intact lung. We show that PNX stimulates pulmonary capillary endothelial cells (PCECs) to produce angiocrine growth factors that induce proliferation of epithelial progenitor cells supporting alveologenesis. Endothelial cells trigger expansion of cocultured epithelial cells, forming three-dimensional angiospheres reminiscent of alveolar-capillary sacs. After PNX, endothelial-specific inducible genetic ablation of Vegfr2 and Fgfr1 in mice inhibits production of MMP14, impairing alveolarization. MMP14 promotes expansion of epithelial progenitor cells by unmasking cryptic EGF-like ectodomains that activate the EGF receptor (EGFR). Consistent with this, neutralization of MMP14 impairs EGFR-mediated alveolar regeneration, whereas administration of EGF or intravascular transplantation of MMP14(+) PCECs into pneumonectomized Vegfr2/Fgfr1-deficient mice restores alveologenesis and lung inspiratory volume and compliance function. VEGFR2 and FGFR1 activation in PCECs therefore increases MMP14-dependent bioavailability of EGFR ligands to initiate and sustain alveologenesis.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures
Comment in
-
A breath of fresh air in lung regeneration.Cell. 2011 Oct 28;147(3):485-7. doi: 10.1016/j.cell.2011.10.008. Cell. 2011. PMID: 22036554 Free PMC article.
References
-
- Aird WC. Phenotypic heterogeneity of the endothelium: I. Structure, function, and mechanisms. Circ Res. 2007;100:158–173. - PubMed
-
- Alvarez DF, Huang L, King JA, ElZarrad MK, Yoder MC, Stevens T. Lung microvascular endothelium is enriched with progenitor cells that exhibit vasculogenic capacity. Am J Physiol Lung Cell Mol Physiol. 2008;294:L419–430. - PubMed
-
- Atkinson JJ, Holmbeck K, Yamada S, Birkedal-Hansen H, Parks WC, Senior RM. Membrane-type 1 matrix metalloproteinase is required for normal alveolar development. Dev Dyn. 2005;232:1079–1090. - PubMed
-
- Beers MF, Kim CY, Dodia C, Fisher AB. Localization, synthesis, and processing of surfactant protein SP-C in rat lung analyzed by epitope-specific antipeptide antibodies. J Biol Chem. 1994;269:20318–20328. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

