Altered modes of stem cell division drive adaptive intestinal growth
- PMID: 22036568
- PMCID: PMC3246009
- DOI: 10.1016/j.cell.2011.08.048
Altered modes of stem cell division drive adaptive intestinal growth
Abstract
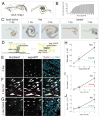
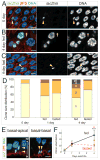
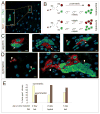
Throughout life, adult organs continually adapt to variable environmental factors. Adaptive mechanisms must fundamentally differ from homeostatic maintenance, but little is known about how physiological factors elicit tissue remodeling. Here, we show that specialized stem cell responses underlie the adaptive resizing of a mature organ. In the adult Drosophila midgut, intestinal stem cells interpret a nutrient cue to "break homeostasis" and drive growth when food is abundant. Activated in part by niche production of insulin, stem cells direct a growth program through two altered modes of behavior: accelerated division rates and predominance of symmetric division fates. Together, these altered modes produce a net increase in total intestinal cells, which is reversed upon withdrawal of food. Thus, tissue renewal programs are not committed to maintain cellular equilibrium; stem cells can remodel organs in response to physiological triggers.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures




Comment in
-
A gutsy way to grow: intestinal stem cells as nutrient sensors.Cell. 2011 Oct 28;147(3):487-9. doi: 10.1016/j.cell.2011.10.006. Cell. 2011. PMID: 22036555
-
Stem cells: having the guts to grow.Nat Rev Mol Cell Biol. 2011 Nov 23;12(12):768. doi: 10.1038/nrm3233. Nat Rev Mol Cell Biol. 2011. PMID: 22108594 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

