Motor neuron position and topographic order imposed by β- and γ-catenin activities
- PMID: 22036570
- PMCID: PMC3226777
- DOI: 10.1016/j.cell.2011.09.037
Motor neuron position and topographic order imposed by β- and γ-catenin activities
Abstract
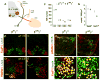
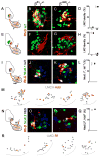
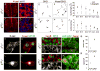
Neurons typically settle at positions that match the location of their synaptic targets, creating topographic maps. In the spinal cord, the organization of motor neurons into discrete clusters is linked to the location of their muscle targets, establishing a topographic map of punctate design. To define the significance of motor pool organization for neuromuscular map formation, we assessed the role of cadherin-catenin signaling in motor neuron positioning and limb muscle innervation. We find that joint inactivation of β- and γ-catenin scrambles motor neuron settling position in the spinal cord but fails to erode the predictive link between motor neuron transcriptional identity and muscle target. Inactivation of N-cadherin perturbs pool positioning in similar ways, albeit with reduced penetrance. These findings reveal that cadherin-catenin signaling directs motor pool patterning and imposes topographic order on an underlying identity-based neural map.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures




References
-
- Brault V, Moore R, Kutsch S, Ishibashi M, Rowitch DH, McMahon AP, Sommer L, Boussadia O, Kemler R. Inactivation of the beta-catenin gene by Wnt1-Cre-mediated deletion results in dramatic brain malformation and failure of craniofacial development. Development. 2001;128:1253–1264. - PubMed
-
- Butz S, Stappert J, Weissig H, Kemler R. Plakoglobin and beta-catenin: distinct but closely related. Science. 1992;257:1142–1144. - PubMed
-
- Dasen JS, De Camilli A, Wang B, Tucker PW, Jessell TM. Hox repertoires for motor neuron diversity and connectivity gated by a single accessory factor, FoxP1. Cell. 2008;134:304–316. - PubMed
-
- Dessaud E, Yang LL, Hill K, Cox B, Ulloa F, Ribeiro A, Mynett A, Novitch BG, Briscoe J. Interpretation of the sonic hedgehog morphogen gradient by a temporal adaptation mechanism. Nature. 2007;450:717–720. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

