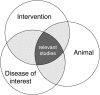
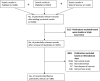
A step-by-step guide to systematically identify all relevant animal studies
- PMID: 22037056
- PMCID: PMC3265183
- DOI: 10.1258/la.2011.011087
A step-by-step guide to systematically identify all relevant animal studies
Abstract
Before starting a new animal experiment, thorough analysis of previously performed experiments is essential from a scientific as well as from an ethical point of view. The method that is most suitable to carry out such a thorough analysis of the literature is a systematic review (SR). An essential first step in an SR is to search and find all potentially relevant studies. It is important to include all available evidence in an SR to minimize bias and reduce hampered interpretation of experimental outcomes. Despite the recent development of search filters to find animal studies in PubMed and EMBASE, searching for all available animal studies remains a challenge. Available guidelines from the clinical field cannot be copied directly to the situation within animal research, and although there are plenty of books and courses on searching the literature, there is no compact guide available to search and find relevant animal studies. Therefore, in order to facilitate a structured, thorough and transparent search for animal studies (in both preclinical and fundamental science), an easy-to-use, step-by-step guide was prepared and optimized using feedback from scientists in the field of animal experimentation. The step-by-step guide will assist scientists in performing a comprehensive literature search and, consequently, improve the scientific quality of the resulting review and prevent unnecessary animal use in the future.
Figures
Similar articles
-
Health professionals' experience of teamwork education in acute hospital settings: a systematic review of qualitative literature.JBI Database System Rev Implement Rep. 2016 Apr;14(4):96-137. doi: 10.11124/JBISRIR-2016-1843. JBI Database System Rev Implement Rep. 2016. PMID: 27532314
-
What is the value of routinely testing full blood count, electrolytes and urea, and pulmonary function tests before elective surgery in patients with no apparent clinical indication and in subgroups of patients with common comorbidities: a systematic review of the clinical and cost-effective literature.Health Technol Assess. 2012 Dec;16(50):i-xvi, 1-159. doi: 10.3310/hta16500. Health Technol Assess. 2012. PMID: 23302507 Free PMC article.
-
Home treatment for mental health problems: a systematic review.Health Technol Assess. 2001;5(15):1-139. doi: 10.3310/hta5150. Health Technol Assess. 2001. PMID: 11532236
-
Population-based interventions for reducing sexually transmitted infections, including HIV infection.Cochrane Database Syst Rev. 2004;(2):CD001220. doi: 10.1002/14651858.CD001220.pub2. Cochrane Database Syst Rev. 2004. Update in: Cochrane Database Syst Rev. 2011 Mar 16;(3):CD001220. doi: 10.1002/14651858.CD001220.pub3. PMID: 15106156 Updated.
-
Eliciting adverse effects data from participants in clinical trials.Cochrane Database Syst Rev. 2018 Jan 16;1(1):MR000039. doi: 10.1002/14651858.MR000039.pub2. Cochrane Database Syst Rev. 2018. PMID: 29372930 Free PMC article.
Cited by
-
Variability of murine bacterial pneumonia models used to evaluate antimicrobial agents.Front Microbiol. 2022 Sep 8;13:988728. doi: 10.3389/fmicb.2022.988728. eCollection 2022. Front Microbiol. 2022. PMID: 36160241 Free PMC article. Review.
-
Are in vitro and in silico approaches used appropriately for animal-based major depressive disorder research?PLoS One. 2020 Jun 24;15(6):e0233954. doi: 10.1371/journal.pone.0233954. eCollection 2020. PLoS One. 2020. PMID: 32579547 Free PMC article.
-
Reviewing the animal literature: how to describe and choose between different types of literature reviews.Lab Anim. 2021 Apr;55(2):129-141. doi: 10.1177/0023677220968599. Epub 2020 Nov 1. Lab Anim. 2021. PMID: 33135562 Free PMC article.
-
Hypotheses and evidence related to intense sweeteners and effects on appetite and body weight changes: A scoping review of reviews.PLoS One. 2018 Jul 18;13(7):e0199558. doi: 10.1371/journal.pone.0199558. eCollection 2018. PLoS One. 2018. PMID: 30020966 Free PMC article.
-
Development of a Systematic Review Protocol and a Scoping Review of Ultrasound-Induced Immune Effects in Peripheral Tumors.Mol Imaging Biol. 2022 Apr;24(2):288-297. doi: 10.1007/s11307-021-01686-x. Epub 2021 Nov 29. Mol Imaging Biol. 2022. PMID: 34845660 Free PMC article.
References
-
- Cook DJ, Mulrow CD, Haynes RB Systematic reviews: synthesis of best evidence for clinical decisions. Ann Intern Med 1997;126:376–80 - PubMed
-
- Chalmers I, Altman D, Systematic Reviews. London: BMJ Publishing Group, 1995
-
- Higgins JPT, Green S Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration; 2011. See www.cochrane-handbook.org
-
- Macleod MR, Fisher M, O'Collins V, et al. Good laboratory practice: preventing introduction of bias at the bench. Stroke 2009;40:e50–2 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials