The mitochondrial transcription and packaging factor Tfam imposes a U-turn on mitochondrial DNA
- PMID: 22037171
- PMCID: PMC3210390
- DOI: 10.1038/nsmb.2159
The mitochondrial transcription and packaging factor Tfam imposes a U-turn on mitochondrial DNA
Abstract
Tfam (transcription factor A, mitochondrial), a DNA-binding protein with tandem high-mobility group (HMG)-box domains, has a central role in the expression, maintenance and organization of the mitochondrial genome. It activates transcription from mitochondrial promoters and organizes the mitochondrial genome into nucleoids. Using X-ray crystallography, we show that human Tfam forces promoter DNA to undergo a U-turn, reversing the direction of the DNA helix. Each HMG-box domain wedges into the DNA minor groove to generate two kinks on one face of the DNA. On the opposite face, a positively charged α-helix serves as a platform to facilitate DNA bending. The structural principles underlying DNA bending converge with those of the unrelated HU family proteins, which have analogous architectural roles in organizing bacterial nucleoids. The functional importance of this extreme DNA bending is promoter specific and seems to be related to the orientation of Tfam on the promoters.
Figures
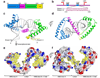
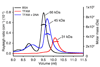

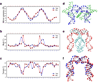
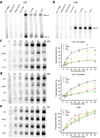
Comment in
-
TFAM forces mtDNA to make a U-turn.Nat Struct Mol Biol. 2011 Nov 4;18(11):1179-81. doi: 10.1038/nsmb.2167. Nat Struct Mol Biol. 2011. PMID: 22056802 No abstract available.
References
Main References
-
- Falkenberg M, Larsson NG, Gustafsson CM. DNA replication and transcription in mammalian mitochondria. Annu Rev Biochem. 2007;76:679–699. - PubMed
-
- Bonawitz ND, Clayton DA, Shadel GS. Initiation and beyond: multiple functions of the human mitochondrial transcription machinery. Mol Cell. 2006;24:813–825. - PubMed
-
- Fisher RP, Topper JN, Clayton DA. Promoter selection in human mitochondria involves binding of a transcription factor to orientation-independent upstream regulatory elements. Cell. 1987;50:247–258. - PubMed
-
- Larsson NG, et al. Mitochondrial transcription factor A is necessary for mtDNA maintenance and embryogenesis in mice. Nat Genet. 1998;18:231–236. - PubMed
Methods-only references
-
- Van Duyne GD, Standaert RF, Karplus PA, Schreiber SL, Clardy J. Atomic structures of the human immunophilin FKBP-12 complexes with FK506 and rapamycin. J Mol Biol. 1993;229:105–124. - PubMed
-
- Leslie AGW. Joint CCP4 and ESF-EACBM Newsletter on Protein Crystallography. Warrington WA4 4AD, UK: 1992.
-
- Evans PR. In: Proceedings of the CCP4 Study Weekend. Data Collection and Processing. Sawyer L, Isaacs N, Bailey S, editors. Warrington: Daresbury Laboratory; 1993. pp. 114–122.
-
- Collaborative Computational Project, N. The CCP4 Suite: Programs for Protein Crystallography. Acta Cryst. 1994;D50:760–763. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

