Vaccine induced antibodies to the first variable loop of human immunodeficiency virus type 1 gp120, mediate antibody-dependent virus inhibition in macaques
- PMID: 22037204
- PMCID: PMC3246802
- DOI: 10.1016/j.vaccine.2011.10.040
Vaccine induced antibodies to the first variable loop of human immunodeficiency virus type 1 gp120, mediate antibody-dependent virus inhibition in macaques
Abstract
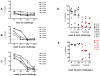
The role of antibodies directed against the hyper variable envelope region V1 of human immunodeficiency virus type 1 (HIV-1), has not been thoroughly studied. We show that a vaccine able to elicit strain-specific non-neutralizing antibodies to this region of gp120 is associated with control of highly pathogenic chimeric SHIV(89.6P) replication in rhesus macaques. The vaccinated animal that had the highest titers of antibodies to the amino terminus portion of V1, prior to challenge, had secondary antibody responses that mediated cell killing by antibody-dependent cellular cytotoxicity (ADCC), as early as 2 weeks after infection and inhibited viral replication by antibody-dependent cell-mediated virus inhibition (ADCVI), by 4 weeks after infection. There was a significant inverse correlation between virus level and binding antibody titers to the envelope protein, (R=-0.83, p=0.015), and ADCVI (R=-0.84 p=0.044). Genotyping of plasma virus demonstrated in vivo selection of three SHIV(89.6P) variants with changes in potential N-linked glycosylation sites in V1. We found a significant inverse correlation between virus levels and titers of antibodies that mediated ADCVI against all the identified V1 virus variants. A significant inverse correlation was also found between neutralizing antibody titers to SHIV(89.6) and virus levels (R=-0.72 p=0.0050). However, passive inoculation of purified immunoglobulin from animal M316, the macaque that best controlled virus, to a naïve macaque, resulted in a low serum neutralizing antibodies and low ADCVI activity that failed to protect from SHIV(89.6P) challenge. Collectively, while our data suggest that anti-envelope antibodies with neutralizing and non-neutralizing Fc(R-dependent activities may be important in the control of SHIV replication, they also demonstrate that low levels of these antibodies alone are not sufficient to protect from infection.
Published by Elsevier Ltd.
Conflict of interest statement
Figures










References
-
- Kijak GH, McCutchan FE. HIV diversity, molecular epidemiology, and the role of recombination. Curr Infect Dis Rep. 2005 Nov;7(6):480–8. - PubMed
-
- Korber B, Gaschen B, Yusim K, Thakallapally R, Kesmir C, Detours V. Evolutionary and immunological implications of contemporary HIV-1 variation. Br Med Bull. 2001;58:19–42. - PubMed
-
- Kowalski M, Potz J, Basiripour L, et al. Functional regions of the envelope glycoprotein of human immunodeficiency virus type 1. Science. 1987 Sep 11;237(4820):1351–5. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

