Negative regulation of Yap during neuronal differentiation
- PMID: 22037235
- PMCID: PMC3235039
- DOI: 10.1016/j.ydbio.2011.10.017
Negative regulation of Yap during neuronal differentiation
Abstract
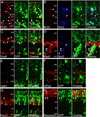
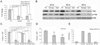
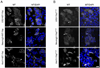
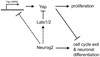
Regulated proliferation and cell cycle exit are essential aspects of neurogenesis. The Yap transcriptional coactivator controls proliferation in a variety of tissues during development, and this activity is negatively regulated by kinases in the Hippo signaling pathway. We find that Yap is expressed in mitotic mouse retinal progenitors and it is downregulated during neuronal differentiation. Forced expression of Yap prolongs proliferation in the postnatal mouse retina, whereas inhibition of Yap by RNA interference (RNAi) decreases proliferation and increases differentiation. We show Yap is subject to post-translational inhibition in the retina, and also downregulated at the level of mRNA expression. Using a cell culture model, we find that expression of the proneural basic helix-loop-helix (bHLH) transcription factors Neurog2 or Ascl1 downregulates Yap mRNA levels, and simultaneously inhibits Yap protein via activation of the Lats1 and/or Lats2 kinases. Conversely, overexpression of Yap prevents proneural bHLH proteins from initiating cell cycle exit. We propose that mutual inhibition between proneural bHLH proteins and Yap is an important regulator of proliferation and cell cycle exit during mammalian neurogenesis.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures




References
-
- Akagi T, Inoue T, Miyoshi G, Bessho Y, Takahashi M, Lee JE, Guillemot F, Kageyama R. Requirement of multiple basic helix-loop-helix genes for retinal neuronal subtype specification. J Biol Chem. 2004;279:28492–28498. - PubMed
-
- Basu S, Totty NF, Irwin MS, Sudol M, Downward J. Akt phosphorylates the Yes-associated protein, YAP, to induce interaction with 14-3-3 and attenuation of p73-mediated apoptosis. Mol Cell. 2003;11:11–23. - PubMed
-
- Ben-Arie N, Bellen HJ, Armstrong DL, McCall AE, Gordadze PR, Guo Q, Matzuk MM, Zoghbi HY. Math1 is essential for genesis of cerebellar granule neurons. Nature. 1997;390:169–172. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

