Domain-based assays of individual antibody concentrations in an oligoclonal combination targeting a single protein
- PMID: 22037290
- PMCID: PMC4209596
- DOI: 10.1016/j.ab.2011.09.030
Domain-based assays of individual antibody concentrations in an oligoclonal combination targeting a single protein
Abstract
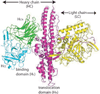
Quantitation of individual monoclonal antibodies (mAbs) within a combined antibody drug product is required for preclinical and clinical drug development, including pharmacokinetic (PK), toxicology, stability, and biochemical characterization studies of such drugs. We have developed an antitoxin, XOMA 3AB, consisting of three recombinant mAbs that potently neutralize the known subtypes of type A botulinum neurotoxin (BoNT/A). The three mAbs bind nonoverlapping BoNT/A epitopes with high affinity. XOMA 3AB is being developed as a treatment for botulism resulting from BoNT/A. To develop antibody-specific assays, we cloned, expressed, and purified BoNT/A domains from Escherichia coli. Each mAb bound only to its specific domain with affinity comparable to the binding to holotoxin. mAb-specific domains were used to develop an enzyme-linked immunosorbent assay (ELISA) for characterization of the integrity and binding activity of the three mAbs in the drug product. An electrochemiluminescence bridging assay that is robust to interference from components in serum was also developed, and we demonstrate that it can be used for PK assays. This type of antigen engineering to generate mAb-specific domains is a general method allowing quantitation and characterization of individual mAbs in a mAb cocktail that binds the same protein and is superior to anti-idiotype approaches.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures





References
-
- Nelson AL, Dhimolea E, Reichert JM. Development trends for human monoclonal antibody therapeutics. Nature Reviews Drug Discovery. 2010;9(10):767–774. - PubMed
-
- Logtenberg T. Antibody cocktails: next-generation biopharmaceuticals with improved potency. Trends Biotechnol. 2007;25(9):390–394. - PubMed
-
- Bakker AB, et al. First administration to humans of a monoclonal antibody cocktail against rabies virus: safety, tolerability, and neutralizing activity. Vaccine. 2008;26(47):5922–5927. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

