Modulation of dADAR-dependent RNA editing by the Drosophila fragile X mental retardation protein
- PMID: 22037499
- PMCID: PMC3225737
- DOI: 10.1038/nn.2950
Modulation of dADAR-dependent RNA editing by the Drosophila fragile X mental retardation protein
Abstract
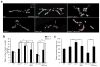
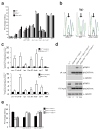
Loss of FMR1 gene function results in fragile X syndrome, the most common heritable form of intellectual disability. The protein encoded by this locus (FMRP) is an RNA-binding protein that is thought to primarily act as a translational regulator; however, recent studies have implicated FMRP in other mechanisms of gene regulation. We found that the Drosophila fragile X homolog (dFMR1) biochemically interacted with the adenosine-to-inosine RNA-editing enzyme dADAR. Adar and Fmr1 mutant larvae exhibited distinct morphological neuromuscular junction (NMJ) defects. Epistasis experiments based on these phenotypic differences revealed that Adar acts downstream of Fmr1 and that dFMR1 modulates dADAR activity. Furthermore, sequence analyses revealed that a loss or overexpression of dFMR1 affects editing efficiency on certain dADAR targets with defined roles in synaptic transmission. These results link dFMR1 with the RNA-editing pathway and suggest that proper NMJ synaptic architecture requires modulation of dADAR activity by dFMR1.
Conflict of interest statement
The authors declare no financial conflict of interest.
Figures
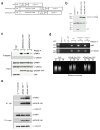
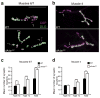
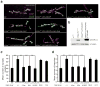
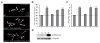
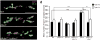
Comment in
-
Fragile balance: RNA editing tunes the synapse.Nat Neurosci. 2011 Nov 23;14(12):1492-4. doi: 10.1038/nn.2982. Nat Neurosci. 2011. PMID: 22119945 No abstract available.
References
-
- O’Donnell WT, Warren ST. A decade of molecular studies of fragile X syndrome. Annu Rev Neurosci. 2002;25:315–338. - PubMed
-
- Feng Y, et al. FMRP associates with polyribosomes as an mRNP, and the I304N mutation of severe fragile X syndrome abolishes this association. Mol Cell. 1997;1:109–118. - PubMed
-
- Corbin F, et al. The fragile X mental retardation protein is associated with poly(A)+ mRNA in actively translating polyribosomes. Hum Mol Genet. 1997;6:1465–1472. - PubMed
-
- Khandjian EW, Corbin F, Woerly S, Rousseau F. The fragile X mental retardation protein is associated with ribosomes. Nat Genet. 1996;12:91–93. - PubMed
-
- Eberhart DE, Malter HE, Feng Y, Warren ST. The fragile X mental retardation protein is a ribonucleoprotein containing both nuclear localization and nuclear export signals. Hum Mol Genet. 1996;5:1083–1091. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

