Compromised mitochondrial complex II in models of Machado-Joseph disease
- PMID: 22037589
- PMCID: PMC3338188
- DOI: 10.1016/j.bbadis.2011.10.010
Compromised mitochondrial complex II in models of Machado-Joseph disease
Abstract
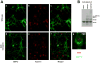
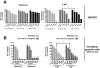
Machado-Joseph disease (MJD), also known as Spinocerebellar Ataxia type 3, is an inherited dominant autosomal neurodegenerative disorder. An expansion of Cytosine-Adenine-Guanine (CAG) repeats in the ATXN3 gene is translated as an expanded polyglutamine domain in the disease protein, ataxin-3. Selective neurodegeneration in MJD is evident in several subcortical brain regions including the cerebellum. Mitochondrial dysfunction has been proposed as a mechanism of neurodegeneration in polyglutamine disorders. In this study, we used different cell models and transgenic mice to assess the importance of mitochondria on cytotoxicity observed in MJD. Transiently transfected HEK cell lines with expanded (Q84) ataxin-3 exhibited a higher susceptibility to 3-nitropropionic acid (3-NP), an irreversible inhibitor of mitochondrial complex II. Increased susceptibility to 3-NP was also detected in stably transfected PC6-3 cells that inducibly express expanded (Q108) ataxin-3 in a tetracycline-regulated manner. Moreover, cerebellar granule cells from MJD transgenic mice were more sensitive to 3-NP inhibition than wild-type cerebellar neurons. PC6-3 (Q108) cells differentiated into a neuronal-like phenotype with nerve growth factor (NGF) exhibited a significant decrease in mitochondrial complex II activity. Mitochondria from MJD transgenic mouse model and lymphoblast cell lines derived from MJD patients also showed a trend toward reduced complex II activity. Our results suggest that mitochondrial complex II activity is moderately compromised in MJD, which may designate a common feature in polyglutamine toxicity.
Copyright © 2011 Elsevier B.V. All rights reserved.
Figures


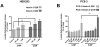

References
-
- Albrecht M, Hoffman D, Evert BO, Scmitt I, Wullner U, Lengauer T. Structural modelling of ataxin-3 reveals distant homology to adaptins. Proteins. 2003;50:355–370. - PubMed
-
- Rüb U, Brunt ER, Del Turco D, de Vos RA, Gierga K, Paulson H, Braak H. The nucleus raphe interpositus in spinocerebellar ataxia type 3 (Machado-Joseph disease) J Chem Neuroanat. 2003;25:115–127. - PubMed
-
- Rüb U, de Vos RA, Schultz C, Brunt ER, Paulson H, Braak H. Spinocerebellar ataxia type 3 (Machado-Joseph disease): severe destruction of the lateral reticular nucleus. Brain. 2002;125:2115–2124. - PubMed
-
- Kawagushi Y, Okamoto T, Taniwaki M, Aizawa M, Inoue M, Katayama S, Kawakami H, Nakamura S, Nishimura M, Akiguchi I, et al. CAG expansions in a novel gene for Machado-Joseph disease at chromosome 14q32.1. Nat Genet. 1994;8:221–8. - PubMed
-
- Riess O, Rub U, Pastore A, Bauer P, Schols L. SCA3: Neurological features, pathogenesis and animal models. Cerebellum. 2008;7:125–137. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

