Potential role for alternatively activated macrophages in the secondary bacterial infection during recovery from influenza
- PMID: 22037624
- PMCID: PMC3243824
- DOI: 10.1016/j.imlet.2011.10.009
Potential role for alternatively activated macrophages in the secondary bacterial infection during recovery from influenza
Abstract
Purpose: Secondary bacterial infections are a common complication of influenza. Innate immune host defenses appear to be impaired following influenza, leading to susceptibility to subsequent bacterial infections. Alternatively activated macrophages (AAM) in the lungs may play a critical role in eliciting the hypersusceptibility to secondary bacterial pneumonia.
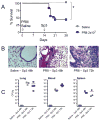
Methods: C57BL6 mice were challenged with sublethal doses of the mouse-adapted A/PR/8/34 (PR8) influenza virus or saline and allowed to recover. At complete recovery (day 14), mice were re-challenged with sublethal doses of Streptococcus pneumoniae serotype 3 (Sp3).
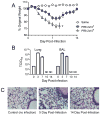
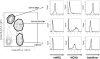
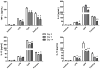
Results: PR8-recovered mice developed a rapidly fatal pulmonary infection to a 100-fold sublethal pneumococcal challenge, whereas PR8-naive mice demonstrated no mortality or illness. The cytokines which induce AAM (IL-4 and IL-13) and the expression of genes associated with AAM (Arginase-1, FIZZ1, and YM1) were elevated after PR8 infection. Flow cytometry suggests that alveolar macrophages demonstrate the AAM-phenotype, as indicated by MGL-1 and MHCII expression, in response to PR8 infection. Recovery from PR8 was associated with blunted cytokine responses to TLR ligands.
Conclusions: The mechanisms of immune regulation during recovery from influenza are being elucidated. We provide evidence that pulmonary AAM are induced during influenza infection and may contribute to the elicitation of hypersusceptibility to a secondary bacterial infection.
Copyright © 2011 Elsevier B.V. All rights reserved.
Figures


References
-
- HERS JF, MASUREL N, MULDER J. Bacteriology and histopathology of the respiratory tract and lungs in fatal Asian influenza. Lancet. 1958;2:1141–1143. - PubMed
-
- Lindsay MI, Jr, Herrmann EC, Jr, Morrow GW, Jr, Brown AL., Jr Hong Kong influenza: clinical, microbiologic, and pathologic features in 127 cases. JAMA. 1970;214:1825–1832. - PubMed
-
- Kashiwagi S, Ikematsu H, Hayashi J, Nomura H, Kajiyama W, Kaji M. An outbreak of influenza A (H3N2) in a hospital for the elderly with emphasis on pulmonary complications. Jpn J Med. 1988;27:177–182. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

