Perioperative pharmacokinetics of methadone in adolescents
- PMID: 22037641
- PMCID: PMC3560937
- DOI: 10.1097/ALN.0b013e318238fec5
Perioperative pharmacokinetics of methadone in adolescents
Abstract
Background: Methadone is frequently administered to adults experiencing anesthesia and receiving pain treatment. Methadone pharmacokinetics in adults are well characterized, including the perioperative period. Methadone is also used in children. There is, however, no information on methadone pharmacokinetics in children of any age. The purpose of this investigation was to determine the pharmacokinetics of intravenous methadone in children undergoing surgery. Perioperative opioid-sparing effects were also assessed.
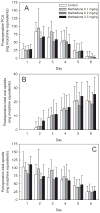
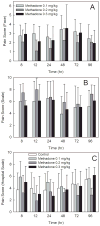
Methods: Eligible subjects were children 5-18 yr undergoing general anesthesia and surgery, with an anticipated postoperative inpatient stay exceeding 3 days. Three groups of 10 to 11 patients each received intravenous methadone hydrochloride after anesthetic induction in ascending dose groups of 0.1, 0.2, and 0.3 mg/kg (up to 20 mg). Anesthetic care was not otherwise changed. Venous blood was obtained for 4 days, for stereoselective determination of methadone and metabolites. Pain assessments were made each morning. Daily and total opioid consumption was determined. Perioperative opioid consumption and pain was determined in a second cohort, which was matched to age, sex, race, ethnicity, surgical procedure, and length of stay, but not receiving methadone.
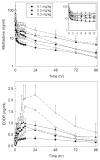
Results: The final methadone study cohort was 31 adolescents (14 ± 2 yr, range 10-18) undergoing major spine surgery for a diagnosis of scoliosis. Methadone pharmacokinetics were linear over the dose range 0.1-0.3 mg/kg. Disposition was stereoselective. Methadone administration did not dose-dependently affect postoperative pain scores, and did not dose-dependently decrease daily or total postoperative opioid consumption in spinal fusion patients.
Conclusions: Methadone enantiomer disposition in adolescents undergoing surgery was similar to that in healthy adults.
Figures


Comment in
-
Perioperative role of methadone in adolescent patients.Anesthesiology. 2012 Jun;116(6):1400-1; author reply 1401. doi: 10.1097/ALN.0b013e318255795d. Anesthesiology. 2012. PMID: 22617207 No abstract available.
References
-
- Kokki H. Current management of pediatric postoperative pain. Expert Rev Neurother. 2004;4:295–306. - PubMed
-
- Fortier MA, MacLaren JE, Martin SR, Perret-Karimi D, Kain ZN. Pediatric pain after ambulatory surgery: Where’s the medication? Pediatrics. 2009;124:e588–95. - PubMed
-
- Rony RY, Fortier MA, Chorney JM, Perret D, Kain ZN. Parental postoperative pain management: Attitudes, assessment, and management. Pediatrics. 2010;125:e1372–8. - PubMed
-
- Monitto CL, Kost-Byerly S, Yaster M. In: Pain management, Anesthesia for infants and children. Davis PJ, Cladis FP, Motoyama EK, editors. Philadelphia: Elsevier-Mosby; 2011. pp. 418–51.
-
- Zempsky WT, Cravero JP. Relief of pain and anxiety in pediatric patients in emergency medical systems. Pediatrics. 2004;114:1348–56. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

