Neurexins and neuroligins: recent insights from invertebrates
- PMID: 22037798
- PMCID: PMC3229692
- DOI: 10.1007/s12035-011-8213-1
Neurexins and neuroligins: recent insights from invertebrates
Abstract
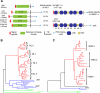
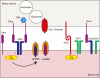
During brain development, each neuron must find and synapse with the correct pre- and postsynaptic partners. The complexity of these connections and the relatively large distances some neurons must send their axons to find the correct partners makes studying brain development one of the most challenging, and yet fascinating disciplines in biology. Furthermore, once the initial connections have been made, the neurons constantly remodel their dendritic and axonal arbours in response to changing demands. Neurexin and neuroligin are two cell adhesion molecules identified as important regulators of this process. The importance of these genes in the development and modulation of synaptic connectivity is emphasised by the observation that mutations in these genes in humans have been associated with cognitive disorders such as Autism spectrum disorders, Tourette syndrome and Schizophrenia. The present review will discuss recent advances in our understanding of the role of these genes in synaptic development and modulation, and in particular, we will focus on recent work in invertebrate models, and how these results relate to studies in mammals.
Figures
References
-
- Chih B, Engelman H, Scheiffele P. Control of excitatory and inhibitory synapse formation by neuroligins. Science. 2005;307:1324–1328. - PubMed
-
- Scheiffele P, Fan J, Choih J, Fetter R, Serafini T. Neuroligin expressed in nonneuronal cells triggers presynaptic development in contacting axons. Cell. 2000;101:657–669. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

