Crystal structure of a heterodimer of editosome interaction proteins in complex with two copies of a cross-reacting nanobody
- PMID: 22039098
- PMCID: PMC3287191
- DOI: 10.1093/nar/gkr867
Crystal structure of a heterodimer of editosome interaction proteins in complex with two copies of a cross-reacting nanobody
Abstract
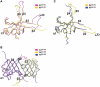
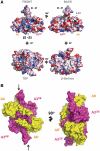
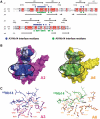
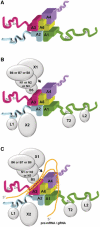
The parasite Trypanosoma brucei, the causative agent of sleeping sickness across sub-Saharan Africa, depends on a remarkable U-insertion/deletion RNA editing process in its mitochondrion. A approximately 20 S multi-protein complex, called the editosome, is an essential machinery for editing pre-mRNA molecules encoding the majority of mitochondrial proteins. Editosomes contain a common core of twelve proteins where six OB-fold interaction proteins, called A1-A6, play a crucial role. Here, we report the structure of two single-strand nucleic acid-binding OB-folds from interaction proteins A3 and A6 that surprisingly, form a heterodimer. Crystal growth required the assistance of an anti-A3 nanobody as a crystallization chaperone. Unexpectedly, this anti-A3 nanobody binds to both A3(OB) and A6, despite only ~40% amino acid sequence identity between the OB-folds of A3 and A6. The A3(OB)-A6 heterodimer buries 35% more surface area than the A6 homodimer. This is attributed mainly to the presence of a conserved Pro-rich loop in A3(OB). The implications of the A3(OB)-A6 heterodimer, and of a dimer of heterodimers observed in the crystals, for the architecture of the editosome are profound, resulting in a proposal of a 'five OB-fold center' in the core of the editosome.
Figures


References
-
- Fairlamb AH. Chemotherapy of human African trypanosomiasis: current and future prospects. Trends. Parasitol. 2003;19:488–494. - PubMed
-
- Hotez PJ, Molyneux DH, Fenwick A, Kumaresan J, Sachs SE, Sachs JD, Savioli L. Control of neglected tropical diseases. N. Engl. J. Med. 2007;357:1018–1027. - PubMed
-
- Moyersoen J, Choe J, Fan E, Hol WG, Michels PA. Biogenesis of peroxisomes and glycosomes: trypanosomatid glycosome assembly is a promising new drug target. FEMS Microbiol. Rev. 2004;28:603–643. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

