Conformational dynamics of helix 8 in the GPCR rhodopsin controls arrestin activation in the desensitization process
- PMID: 22039220
- PMCID: PMC3219140
- DOI: 10.1073/pnas.1015461108
Conformational dynamics of helix 8 in the GPCR rhodopsin controls arrestin activation in the desensitization process
Abstract
Arrestins are regulatory molecules for G-protein coupled receptor function. In visual rhodopsin, selective binding of arrestin to the cytoplasmic side of light-activated, phosphorylated rhodopsin (P-Rh*) terminates signaling via the G-protein transducin. While the "phosphate-sensor" of arrestin for the recognition of receptor-attached phosphates is identified, the molecular mechanism of arrestin binding and the involvement of receptor conformations in this process are still largely hypothetic. Here we used fluorescence pump-probe and time-resolved fluorescence depolarization measurements to investigate the kinetics of arrestin conformational changes and the corresponding nanosecond dynamical changes at the receptor surface. We show that at least two sequential conformational changes of arrestin occur upon interaction with P-Rh*, thus providing a kinetic proof for the suggested multistep nature of arrestin binding. At the cytoplasmic surface of P-Rh*, the structural dynamics of the amphipathic helix 8 (H8), connecting transmembrane helix 7 and the phosphorylated C-terminal tail, depends on the arrestin interaction state. We find that a high mobility of H8 is required in the low-affinity (prebinding) but not in the high-affinity binding state. High-affinity arrestin binding is inhibited when a bulky, inflexible group is bound to H8, indicating close interaction. We further show that this close steric interaction of H8 with arrestin is mandatory for the transition from prebinding to high-affinity binding; i.e., for arrestin activation. This finding implies a regulatory role for H8 in activation of visual arrestin, which shows high selectivity to P-Rh* in contrast to the broad receptor specificity displayed by the two nonvisual arrestins.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

 (red), and three phosphorylated serines, p-S334, p-S338, and p-S343 (blue). Dark yellow- residues belonging to the NPxxY motif. Green- side chains of the hydrophobic residues F313, M317, and L321. (D) Chemical structure of 5-IAF (SF) and Alexa594 (LF) bound to C316.
(red), and three phosphorylated serines, p-S334, p-S338, and p-S343 (blue). Dark yellow- residues belonging to the NPxxY motif. Green- side chains of the hydrophobic residues F313, M317, and L321. (D) Chemical structure of 5-IAF (SF) and Alexa594 (LF) bound to C316.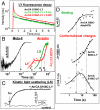

 in percentage (
in percentage ( ), (I) steric restriction r∞ of H8. Black—dark (inactive) rhodopsin, red—light-activated rhodopsin, blue—phosphorylated rhodopsin, green—presence of arrestin. Conditions: 5 μM rhodopsin, 50 μM arrestin, 150 mM NaCl, 50 mM potassium phosphate buffer pH 7.5, 20 °C. The fluorescence excitation was at 470 nm and the emission was detected after passing through a cut-off filter OG515.
), (I) steric restriction r∞ of H8. Black—dark (inactive) rhodopsin, red—light-activated rhodopsin, blue—phosphorylated rhodopsin, green—presence of arrestin. Conditions: 5 μM rhodopsin, 50 μM arrestin, 150 mM NaCl, 50 mM potassium phosphate buffer pH 7.5, 20 °C. The fluorescence excitation was at 470 nm and the emission was detected after passing through a cut-off filter OG515.
References
-
- Pierce KL, Premont RT, Lefkowitz RJ. Seven-transmembrane receptors. Nat Rev Mol Cell Biol. 2002;3:639–650. - PubMed
-
- Gurevich VV, et al. Arrestin interactions with G protein-coupled receptors. Direct binding studies of wild type and mutant arrestins with rhodopsin, beta 2-adrenergic, and m2 muscarinic cholinergic receptors. J Biol Chem. 1995;270:720–731. - PubMed
-
- Goodman OB, Jr, et al. Beta-arrestin acts as a clathrin adaptor in endocytosis of the beta2-adrenergic receptor. Nature. 1996;383:447–450. - PubMed
-
- Lefkowitz RJ, Shenoy SK. Transduction of receptor signals by beta-arrestins. Science. 2005;308:512–517. - PubMed
-
- Sakmar TP. Structure of rhodopsin and the superfamily of seven-helical receptors: the same and not the same. Curr Opin Cell Biol. 2002;14:189–195. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

