Biologic agents for rheumatoid arthritis--negotiating the NICE technology appraisals
- PMID: 22039226
- PMCID: PMC3276292
- DOI: 10.1093/rheumatology/ker321
Biologic agents for rheumatoid arthritis--negotiating the NICE technology appraisals
Abstract
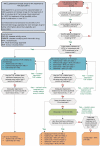
In England and Wales, the National Institute for Health and Clinical Excellence (NICE) has provided guidance [technology appraisals (TAs) 130, 186, 195, 198 and 225] on the use of biologic drugs for the treatment of RA. This is based on an analysis of efficacy, safety and cost-effectiveness, and has resulted in a complex management pathway that restricts freedom to prescribe biologics according to their licensed indications. Specifically, TNF antagonists are the only class of biologics that can be used first line in DMARD-inadequate responders, and only in patients with a persistent 28-joint DAS score of ≥5.1. Alternative biologic agents are denied to those with contraindications to anti-TNF drugs and are also not supported following intolerance to TNF antagonists. Rituximab is the only class of biologic permitted after TNF antagonist inefficacy, in the absence of a contraindication to its use, whereas abatacept and tocilizumab are licensed and may be a more efficacious choice at this stage in some patient groups. Furthermore, for patients who demonstrate sequential inadequate responses, treatment is restricted to one TNF antagonist, rituximab and tocilizumab, whereas abatacept is only a permitted choice when rituximab is contraindicated or has been withdrawn because of an adverse event. In this review, we discuss the treatment algorithm published by NICE, and suggest alternatives where perceived deficiencies exist.
Figures
References
-
- Kiely PDW, Brown AK, Edwards CJ, et al. Contemporary treatment principles for early rheumatoid arthritis: a consensus statement. Rheumatology. 2009;48:765–72. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases