Mechanism of disruption of the Amt-GlnK complex by P(II)-mediated sensing of 2-oxoglutarate
- PMID: 22039461
- PMCID: PMC3198391
- DOI: 10.1371/journal.pone.0026327
Mechanism of disruption of the Amt-GlnK complex by P(II)-mediated sensing of 2-oxoglutarate
Abstract
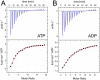
GlnK proteins regulate the active uptake of ammonium by Amt transport proteins by inserting their regulatory T-loops into the transport channels of the Amt trimer and physically blocking substrate passage. They sense the cellular nitrogen status through 2-oxoglutarate, and the energy level of the cell by binding both ATP and ADP with different affinities. The hyperthermophilic euryarchaeon Archaeoglobus fulgidus possesses three Amt proteins, each encoded in an operon with a GlnK ortholog. One of these proteins, GlnK2 was recently found to be incapable of binding 2-OG, and in order to understand the implications of this finding we conducted a detailed structural and functional analysis of a second GlnK protein from A. fulgidus, GlnK3. Contrary to Af-GlnK2 this protein was able to bind both ATP/2-OG and ADP to yield inactive and functional states, respectively. Due to the thermostable nature of the protein we could observe the exact positioning of the notoriously flexible T-loops and explain the binding behavior of GlnK proteins to their interaction partner, the Amt proteins. A thermodynamic analysis of these binding events using microcalorimetry evaluated by microstate modeling revealed significant differences in binding cooperativity compared to other characterized P(II) proteins, underlining the diversity and adaptability of this class of regulatory signaling proteins.
Conflict of interest statement
Figures
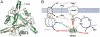
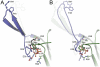
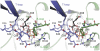


Similar articles
-
Structure of GlnK1, a signalling protein from Archaeoglobus fulgidus.Acta Crystallogr Sect F Struct Biol Cryst Commun. 2011 Feb 1;67(Pt 2):178-81. doi: 10.1107/S1744309110047482. Epub 2011 Jan 21. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2011. PMID: 21301082 Free PMC article.
-
Cooperative binding of MgATP and MgADP in the trimeric P(II) protein GlnK2 from Archaeoglobus fulgidus.J Mol Biol. 2010 Sep 10;402(1):165-77. doi: 10.1016/j.jmb.2010.07.020. Epub 2010 Jul 17. J Mol Biol. 2010. PMID: 20643148
-
Uridylylation of Herbaspirillum seropedicae GlnB and GlnK proteins is differentially affected by ATP, ADP and 2-oxoglutarate in vitro.Arch Microbiol. 2012 Aug;194(8):643-52. doi: 10.1007/s00203-012-0799-9. Epub 2012 Mar 1. Arch Microbiol. 2012. PMID: 22382722
-
Control of AmtB-GlnK complex formation by intracellular levels of ATP, ADP, and 2-oxoglutarate.J Biol Chem. 2010 Oct 1;285(40):31037-45. doi: 10.1074/jbc.M110.153908. Epub 2010 Jul 18. J Biol Chem. 2010. PMID: 20639578 Free PMC article.
-
Structural and functional insights into the AmtB/Mep/Rh protein family.Transfus Clin Biol. 2006 Mar-Apr;13(1-2):65-9. doi: 10.1016/j.tracli.2006.02.014. Epub 2006 Mar 24. Transfus Clin Biol. 2006. PMID: 16564194 Review.
Cited by
-
Similarities in the structure of the transcriptional repressor AmtR in two different space groups suggest a model for the interaction with GlnK.Acta Crystallogr F Struct Biol Commun. 2017 Mar 1;73(Pt 3):146-151. doi: 10.1107/S2053230X17002485. Epub 2017 Feb 21. Acta Crystallogr F Struct Biol Commun. 2017. PMID: 28291750 Free PMC article.
-
From cyanobacteria to plants: conservation of PII functions during plastid evolution.Planta. 2013 Feb;237(2):451-62. doi: 10.1007/s00425-012-1801-0. Epub 2012 Nov 29. Planta. 2013. PMID: 23192387 Review.
-
P(II) signal transduction proteins are ATPases whose activity is regulated by 2-oxoglutarate.Proc Natl Acad Sci U S A. 2013 Aug 6;110(32):12948-53. doi: 10.1073/pnas.1304386110. Epub 2013 Jul 1. Proc Natl Acad Sci U S A. 2013. PMID: 23818625 Free PMC article.
-
Nitrogen regulation of protein-protein interactions and transcript levels of GlnK PII regulator and AmtB ammonium transporter homologs in Archaea.Microbiologyopen. 2013 Oct;2(5):826-40. doi: 10.1002/mbo3.120. Epub 2013 Aug 28. Microbiologyopen. 2013. PMID: 24039236 Free PMC article.
-
Structural basis and target-specific modulation of ADP sensing by the Synechococcus elongatus PII signaling protein.J Biol Chem. 2014 Mar 28;289(13):8960-72. doi: 10.1074/jbc.M113.536557. Epub 2014 Feb 11. J Biol Chem. 2014. PMID: 24519945 Free PMC article.
References
-
- Andrade SL, Einsle O. The Amt/Mep/Rh family of ammonium transport proteins. Mol Membr Biol. 2007;24:357–365. - PubMed
-
- Khademi S, O'Connell J, 3rd, Remis J, Robles-Colmenares Y, Miercke LJ, et al. Mechanism of ammonia transport by Amt/MEP/Rh: structure of AmtB at 1.35 Å. Science. 2004;305:1587–1594. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Miscellaneous

