Targeted deletion of Nrf2 reduces urethane-induced lung tumor development in mice
- PMID: 22039513
- PMCID: PMC3198791
- DOI: 10.1371/journal.pone.0026590
Targeted deletion of Nrf2 reduces urethane-induced lung tumor development in mice
Abstract
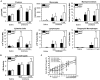
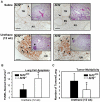
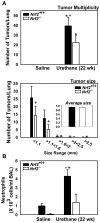
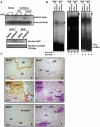
Nrf2 is a key transcription factor that regulates cellular redox and defense responses. However, permanent Nrf2 activation in human lung carcinomas promotes pulmonary malignancy and chemoresistance. We tested the hypothesis that Nrf2 has cell survival properties and lack of Nrf2 suppresses chemically-induced pulmonary neoplasia by treating Nrf2(+/+) and Nrf2(-/-) mice with urethane. Airway inflammation and injury were assessed by bronchoalveolar lavage analyses and histopathology, and lung tumors were analyzed by gross and histologic analysis. We used transcriptomics to assess Nrf2-dependent changes in pulmonary gene transcripts at multiple stages of neoplasia. Lung hyperpermeability, cell death and apoptosis, and inflammatory cell infiltration were significantly higher in Nrf2(-/-) mice compared to Nrf2(+/+) mice 9 and 11 wk after urethane. Significantly fewer lung adenomas were found in Nrf2(-/-) mice than in Nrf2(+/+) mice at 12 and 22 wk. Nrf2 modulated expression of genes involved cell-cell signaling, glutathione metabolism and oxidative stress response, and immune responses during early stage neoplasia. In lung tumors, Nrf2-altered genes had roles in transcriptional regulation of cell cycle and proliferation, carcinogenesis, organismal injury and abnormalities, xenobiotic metabolism, and cell-cell signaling genes. Collectively, Nrf2 deficiency decreased susceptibility to urethane-induced lung tumorigenesis in mice. Cell survival properties of Nrf2 were supported, at least in part, by reduced early death of initiated cells and heightened advantage for tumor cell expansion in Nrf2(+/+) mice relative to Nrf2(-/-) mice. Our results were consistent with the concept that Nrf2 over-activation is an adaptive response of cancer conferring resistance to anti-cancer drugs and promoting malignancy.
Conflict of interest statement
Figures



References
-
- Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. - PubMed
-
- Schottenfeld D. Etiology and Epidemiology of Lung Cancer. In: Pass HI, Mitchell JB, Johnson DH, Turrisi AT, Minna JD, editors. Lung Cancer- Principles and Practice. 3rd ed. Philadelphia: Lippincott Williams and Wilkins; 2005. pp. 3–24.
-
- Colby TV, Noguchi M, Henschke C, Vazquez MF, Geisinger K, Yokose T, et al. Adenocarcinoma. In: Travis WD, Brambilla, E, Muller-Hermelink, H.K, Harris, C.C, editors. World Health Organization classification of tumours: pathology and genetics of tumours of the lung, pleura, thymus, and heart. Lyon, France: IARC Press; 2004. pp. 35–44.
-
- Malkinson AM. Primary lung tumors in mice: an experimentally manipulable model of human adenocarcinoma. Cancer Res. 1992;52:2670s–2676s. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

