Short-term exenatide treatment leads to significant weight loss in a subset of obese women without diabetes
- PMID: 22040840
- PMCID: PMC3241299
- DOI: 10.2337/dc11-0931
Short-term exenatide treatment leads to significant weight loss in a subset of obese women without diabetes
Abstract
Objective: To investigate the effect of treatment with the glucagon-like peptide 1 receptor agonist exenatide on weight loss and metabolic parameters in obese nondiabetic women.
Research design and methods: Forty-one obese women (aged 48 ± 11 years and BMI 33.1 ± 4.1 kg/m(2)) participated in a 35-week randomized, double-blind, placebo-controlled, crossover study, including two 16-week treatment periods separated by a 3-week washout period. There was no lifestyle intervention. The primary outcome was change in body weight.
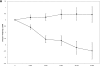
Results: Subjects treated with exenatide lost an average of 2.49 ± 0.66 kg compared with a 0.43 ± 0.63 kg weight gain during placebo treatment. Weight loss with exenatide treatment was noted at 2 weeks. The degree of weight loss could be stratified. A total of 30% of subjects were high responders who lost ≥5% body weight (-7.96 ± 0.52%), 39% were moderate responders who lost <5% body weight (-2.43 ± 0.45%), and 31% were nonresponders who gained weight (1.93 ± 0.53%). Waist circumference also decreased significantly with exenatide treatment. Subjects experienced more nausea during exenatide treatment compared with placebo, but the severity decreased over time and did not correlate with weight loss.
Conclusions: Short-term exenatide treatment was associated with modest weight loss and decreased waist circumference in a cohort of obese nondiabetic women. A subset of individuals demonstrated robust weight loss that was detected very early in the course of treatment.
Figures



References
-
- Shai I, Schwarzfuchs D, Henkin Y, et al. ; Dietary Intervention Randomized Controlled Trial (DIRECT) Group Weight loss with a low-carbohydrate, Mediterranean, or low-fat diet. N Engl J Med 2008;359:229–241 - PubMed
-
- Barte JCM, ter Bogt NCW, Bogers RP, et al. Maintenance of weight loss after lifestyle interventions for overweight and obesity, a systematic review. Obes Rev 2010;11:899–906 - PubMed

