Sirtuins in neurodegenerative diseases: a biological-chemical perspective
- PMID: 22041967
- PMCID: PMC3254029
- DOI: 10.1159/000329724
Sirtuins in neurodegenerative diseases: a biological-chemical perspective
Abstract
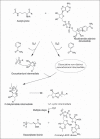
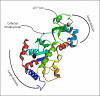
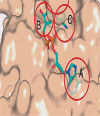
Sirtuins, commonly known as NAD(+)-dependent class III histone deacetylase enzymes, have been extensively studied to evaluate their potential role in different disease states. Based on the published literature, sirtuins have been implicated in providing a myriad of intrinsic and extrinsic biological effects, which in turn may play an important role in the treatment of various disorders such as type II diabetes, obesity, cancer, aging and different neurodegenerative diseases. In particular, a number of studies have unequivocally supported the idea of sirtuins having therapeutic potential in neurodegenerative diseases such as stroke, ischemic brain injury, Alzheimer's disease, Parkinson's disease, Huntington's disease and amyotrophic lateral sclerosis. To exploit the therapeutic potential of sirtuins, their manipulation in terms of development of small-molecule modulators, inhibitors and analogs has increased dramatically since their inception, in both scientific and industrial worlds. Studies on the structure and catalytic core of sirtuins along with chemical mechanisms and substrate specificity have provided important input into the design and synthesis of sirtuin modulators. To study the role of sirtuins in the biological system, it has become extremely important to understand the molecular and chemical structure of sirtuins. In this review, we have discussed the biological role of sirtuins in various neurodegenerative diseases, and also provided an insight into their chemical structure.
Copyright © 2011 S. Karger AG, Basel.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

