Impenetrable barriers or entry portals? The role of cell-cell adhesion during infection
- PMID: 22042617
- PMCID: PMC3206337
- DOI: 10.1083/jcb.201106011
Impenetrable barriers or entry portals? The role of cell-cell adhesion during infection
Erratum in
- J Cell Biol. 2011 Dec 26;195(7):1205
Abstract
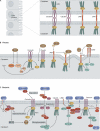
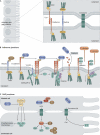
Cell-cell adhesion plays a fundamental role in cell polarity and organogenesis. It also contributes to the formation and establishment of physical barriers against microbial infections. However, a large number of pathogens, from viruses to bacteria and parasites, have developed countless strategies to specifically target cell adhesion molecules in order to adhere to and invade epithelial cells, disrupt epithelial integrity, and access deeper tissues for dissemination. The study of all these processes has contributed to the characterization of molecular machineries at the junctions of eukaryotic cells that have been better understood by using pathogens as probes.
Figures
References
-
- Arbibe L., Kim D.W., Batsché E., Pedron T., Mateescu B., Muchardt C., Parsot C., Sansonetti P.J. 2007. An injected bacterial effector targets chromatin access for transcription factor NF-kappaB to alter transcription of host genes involved in immune responses. Nat. Immunol. 8:47–56 10.1038/ni1423 - DOI - PubMed
-
- Beau I., Cotte-Laffitte J., Amsellem R., Servin A.L. 2007. A protein kinase A-dependent mechanism by which rotavirus affects the distribution and mRNA level of the functional tight junction-associated protein, occludin, in human differentiated intestinal Caco-2 cells. J. Virol. 81:8579–8586 10.1128/JVI.00263-07 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

