In vivo magnetic resonance imaging of the estrogen receptor in an orthotopic model of human breast cancer
- PMID: 22042793
- PMCID: PMC3242887
- DOI: 10.1158/0008-5472.CAN-11-1226
In vivo magnetic resonance imaging of the estrogen receptor in an orthotopic model of human breast cancer
Abstract
Histologic overexpression of the estrogen receptor α (ER) is a well-established prognostic marker in breast cancer. Noninvasive imaging techniques that could detect ER overexpression would be useful in a variety of settings where patients' biopsies are problematic to obtain. This study focused on developing, by in vivo MRI, strategies to measure the level of ER expression in an orthotopic mouse model of human breast cancer. Specifically, novel ER-targeted contrast agents based on pyridine-tetra-acetate-Gd(III) chelate (PTA-Gd) conjugated to 17β-estradiol (EPTA-Gd) or to tamoxifen (TPTA-Gd) were examined in ER-positive or ER-negative tumors. Detection of specific interactions of EPTA-Gd with ER were documented that could differentiate ER-positive and ER-negative tumors. In vivo competition experiments confirmed that the enhanced detection capability of EPTA-Gd was based specifically on ER targeting. In contrast, PTA-Gd acted as an extracellular probe that enhanced ER detection similarly in either tumor type, confirming a similar vascular perfusion efficiency in ER-positive and ER-negative tumors in the model. Finally, TPTA-Gd accumulated selectively in muscle and could not preferentially identify ER-positive tumors. Together, these results define a novel MRI probe that can permit selective noninvasive imaging of ER-positive tumors in vivo.
Figures


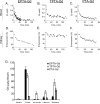
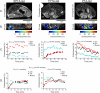

References
-
- Pruthi S, Brandt KR, Degnim AC, Goetz MP, Perez EA, Reynolds CA, et al. A multidisciplinary approach to the management of breast cancer, part 1: prevention and diagnosis. Mayo Clin Proc. 2007;82:999–1012. - PubMed
-
- Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–52. - PubMed
-
- Yang XR, Sherman ME, Rimm DL, Lissowska J, Brinton LA, Peplonska B, et al. Differences in risk factors for breast cancer molecular subtypes in a population-based study. Cancer Epidemiol Biomarkers Prev. 2007;16:439–43. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

