CD47-signal regulatory protein-α (SIRPα) interactions form a barrier for antibody-mediated tumor cell destruction
- PMID: 22042861
- PMCID: PMC3215076
- DOI: 10.1073/pnas.1106550108
CD47-signal regulatory protein-α (SIRPα) interactions form a barrier for antibody-mediated tumor cell destruction
Abstract
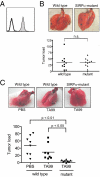
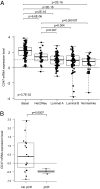
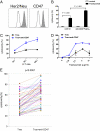
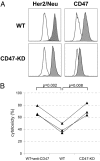
Monoclonal antibodies are among the most promising therapeutic agents for treating cancer. Therapeutic cancer antibodies bind to tumor cells, turning them into targets for immune-mediated destruction. We show here that this antibody-mediated killing of tumor cells is limited by a mechanism involving the interaction between tumor cell-expressed CD47 and the inhibitory receptor signal regulatory protein-α (SIRPα) on myeloid cells. Mice that lack the SIRPα cytoplasmic tail, and hence its inhibitory signaling, display increased antibody-mediated elimination of melanoma cells in vivo. Moreover, interference with CD47-SIRPα interactions by CD47 knockdown or by antagonistic antibodies against CD47 or SIRPα significantly enhances the in vitro killing of trastuzumab-opsonized Her2/Neu-positive breast cancer cells by phagocytes. Finally, the response to trastuzumab therapy in breast cancer patients appears correlated to cancer cell CD47 expression. These findings demonstrate that CD47-SIRPα interactions participate in a homeostatic mechanism that restricts antibody-mediated killing of tumor cells. This provides a rational basis for targeting CD47-SIRPα interactions, using for instance the antagonistic antibodies against human SIRPα described herein, to potentiate the clinical effects of cancer therapeutic antibodies.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Glennie MJ, van de Winkel JG. Renaissance of cancer therapeutic antibodies. Drug Discov Today. 2003;8:503–510. - PubMed
-
- Oldham RK, Dillman RO. Monoclonal antibodies in cancer therapy: 25 years of progress. J Clin Oncol. 2008;26:1774–1777. - PubMed
-
- Strome SE, Sausville EA, Mann D. A mechanistic perspective of monoclonal antibodies in cancer therapy beyond target-related effects. Oncologist. 2007;12:1084–1095. - PubMed
-
- Clynes RA, Towers TL, Presta LG, Ravetch JV. Inhibitory Fc receptors modulate in vivo cytoxicity against tumor targets. Nat Med. 2000;6:443–446. - PubMed
-
- Barok M, et al. Trastuzumab causes antibody-dependent cellular cytotoxicity-mediated growth inhibition of submacroscopic JIMT-1 breast cancer xenografts despite intrinsic drug resistance. Mol Cancer Ther. 2007;6:2065–2072. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

