Quantitative assessment of Plasmodium falciparum sexual development reveals potent transmission-blocking activity by methylene blue
- PMID: 22042867
- PMCID: PMC3223476
- DOI: 10.1073/pnas.1112037108
Quantitative assessment of Plasmodium falciparum sexual development reveals potent transmission-blocking activity by methylene blue
Abstract
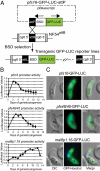
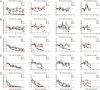
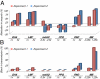
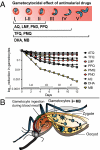
Clinical studies and mathematical models predict that, to achieve malaria elimination, combination therapies will need to incorporate drugs that block the transmission of Plasmodium falciparum sexual stage parasites to mosquito vectors. Efforts to measure the activity of existing antimalarials on intraerythrocytic sexual stage gametocytes and identify transmission-blocking agents have, until now, been hindered by a lack of quantitative assays. Here, we report an experimental system using P. falciparum lines that stably express gametocyte-specific GFP-luciferase reporters, which enable the assessment of dose- and time-dependent drug action on gametocyte maturation and transmission. These studies reveal activity of the first-line antimalarial dihydroartemisinin and the partner drugs lumefantrine and pyronaridine against early gametocyte stages, along with moderate inhibition of mature gametocyte transmission to Anopheles mosquitoes. The other partner agents monodesethyl-amodiaquine and piperaquine showed activity only against immature gametocytes. Our data also identify methylene blue as a potent inhibitor of gametocyte development across all stages. This thiazine dye almost fully abolishes P. falciparum transmission to mosquitoes at concentrations readily achievable in humans, highlighting the potential of this chemical class to reduce the spread of malaria.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

