Gating charge interactions with the S1 segment during activation of a Na+ channel voltage sensor
- PMID: 22042870
- PMCID: PMC3219111
- DOI: 10.1073/pnas.1116449108
Gating charge interactions with the S1 segment during activation of a Na+ channel voltage sensor
Abstract
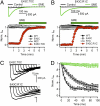
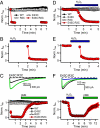
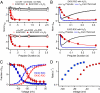
Voltage-gated Na(+) channels initiate action potentials during electrical signaling in excitable cells. Opening and closing of the pore of voltage-gated ion channels are mechanically linked to voltage-driven outward movement of the positively charged S4 transmembrane segment in their voltage sensors. Disulfide locking of cysteine residues substituted for the outermost T0 and R1 gating-charge positions and a conserved negative charge (E43) at the extracellular end of the S1 segment of the bacterial Na(+) channel NaChBac detects molecular interactions that stabilize the resting state of the voltage sensor and define its conformation. Upon depolarization, the more inward gating charges R2 and R3 engage in these molecular interactions as the S4 segment moves outward to its intermediate and activated states. The R4 gating charge does not disulfide-lock with E43, suggesting an outer limit to its transmembrane movement. These molecular interactions reveal how the S4 gating charges are stabilized in the resting state and how their outward movement is catalyzed by interaction with negatively charged residues to effect pore opening and initiate electrical signaling.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

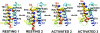
References
-
- Hille B. Ion Channels of Excitable Membranes. 3rd Ed. Sunderland, MA: Sinauer; 2001.
-
- Yu FH, Catterall WA. The VGL-chanome: A protein superfamily specialized for electrical signaling and ionic homeostasis. Sci STKE. 2004;2004:re15. - PubMed
-
- Yi BA, Jan LY. Taking apart the gating of voltage-gated K+ channels. Neuron. 2000;27:423–425. - PubMed
-
- Yellen G. The voltage-gated potassium channels and their relatives. Nature. 2002;419:35–42. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

