Cancer immunotherapy comes of age
- PMID: 22042955
- PMCID: PMC3255990
- DOI: 10.1200/JCO.2011.38.0899
Cancer immunotherapy comes of age
Abstract
Cancer immunotherapy comprises a variety of treatment approaches, incorporating the tremendous specificity of the adaptive immune system (T cells and antibodies) as well as the diverse and potent cytotoxic weaponry of both adaptive and innate immunity. Immunotherapy strategies include antitumor monoclonal antibodies, cancer vaccines, adoptive transfer of ex vivo activated T and natural killer cells, and administration of antibodies or recombinant proteins that either costimulate immune cells or block immune inhibitory pathways (so-called immune checkpoints). Although clear clinical efficacy has been demonstrated with antitumor antibodies since the late 1990s, other immunotherapies had not been shown to be effective until recently, when a spate of successes established the broad potential of this therapeutic modality. These successes are based on fundamental scientific advances demonstrating the toleragenic nature of cancer and the pivotal role of the tumor immune microenvironment in suppressing antitumor immunity. New therapies based on a sophisticated knowledge of immune-suppressive cells, soluble factors, and signaling pathways are designed to break tolerance and reactivate antitumor immunity to induce potent, long-lasting responses. Preclinical models indicate the importance of a complex integrated immune response in eliminating established tumors and validate the exploration of combinatorial treatment regimens, which are anticipated to be far more effective than monotherapies. Unlike conventional cancer therapies, most immunotherapies are active and dynamic, capable of inducing immune memory to propagate a successful rebalancing of the equilibrium between tumor and host.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures
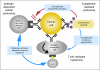



References
-
- Segal NH, Parsons DW, Peggs KS, et al. Epitope landscape in breast and colorectal cancer. Cancer Res. 2008;68:889–892. - PubMed
-
- Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: Integrating immunity's roles in cancer suppression and promotion. Science. 2011;331:1565–1570. - PubMed
-
- McDermott DF, Atkins MB. Application of IL-2 and other cytokines in renal cancer. Expert Opin Biol Ther. 2004;4:455–468. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

