Sequential versus concurrent trastuzumab in adjuvant chemotherapy for breast cancer
- PMID: 22042958
- PMCID: PMC3236650
- DOI: 10.1200/JCO.2011.36.7045
Sequential versus concurrent trastuzumab in adjuvant chemotherapy for breast cancer
Abstract
Purpose: NCCTG (North Central Cancer Treatment Group) N9831 is the only randomized phase III trial evaluating trastuzumab added sequentially or used concurrently with chemotherapy in resected stages I to III invasive human epidermal growth factor receptor 2-positive breast cancer.
Patients and methods: Patients received doxorubicin and cyclophosphamide every 3 weeks for four cycles, followed by paclitaxel weekly for 12 weeks (arm A), paclitaxel plus sequential trastuzumab weekly for 52 weeks (arm B), or paclitaxel plus concurrent trastuzumab for 12 weeks followed by trastuzumab for 40 weeks (arm C). The primary end point was disease-free survival (DFS).
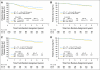
Results: Comparison of arm A (n = 1,087) and arm B (n = 1,097), with 6-year median follow-up and 390 events, revealed 5-year DFS rates of 71.8% and 80.1%, respectively. DFS was significantly increased with trastuzumab added sequentially to paclitaxel (log-rank P < .001; arm B/arm A hazard ratio [HR], 0.69; 95% CI, 0.57 to 0.85). Comparison of arm B (n = 954) and arm C (n = 949), with 6-year median follow-up and 313 events, revealed 5-year DFS rates of 80.1% and 84.4%, respectively. There was an increase in DFS with concurrent trastuzumab and paclitaxel relative to sequential administration (arm C/arm B HR, 0.77; 99.9% CI, 0.53 to 1.11), but the P value (.02) did not cross the prespecified O'Brien-Fleming boundary (.00116) for the interim analysis.
Conclusion: DFS was significantly improved with 52 weeks of trastuzumab added to adjuvant chemotherapy. On the basis of a positive risk-benefit ratio, we recommend that trastuzumab be incorporated into a concurrent regimen with taxane chemotherapy as an important standard-of-care treatment alternative to a sequential regimen.
Trial registration: ClinicalTrials.gov NCT00005970.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures

Comment in
-
Is there an ideal way to combine trastuzumab with chemotherapy?J Clin Oncol. 2011 Dec 1;29(34):4474-6. doi: 10.1200/JCO.2011.38.3836. Epub 2011 Oct 31. J Clin Oncol. 2011. PMID: 22042935 No abstract available.
References
-
- Sjögren S, Inganäs M, Lindgren A, et al. Prognostic and predictive value of c-erbB-2 overexpression in primary breast cancer, alone and in combination with other prognostic markers. J Clin Oncol. 1998;16:462–469. - PubMed
-
- Owens MA, Horten BC, Da Silva MM. HER2 amplification ratios by fluorescence in situ hybridization and correlation with immunohistochemistry in a cohort of 6556 breast cancer tissues. Clin Breast Cancer. 2004;5:63–69. - PubMed
-
- Paik S, Hazan R, Fisher ER, et al. Pathologic findings from the National Surgical Adjuvant Breast and Bowel Project: Prognostic significance of erbB-2 protein overexpression in primary breast cancer. J Clin Oncol. 1990;8:103–112. - PubMed
-
- Gabos Z, Sinha R, Hanson J, et al. Prognostic significance of human epidermal growth factor receptor positivity for the development of brain metastasis after newly diagnosed breast cancer. J Clin Oncol. 2006;24:5658–5663. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

