Longer-term outcomes of letrozole versus placebo after 5 years of tamoxifen in the NCIC CTG MA.17 trial: analyses adjusting for treatment crossover
- PMID: 22042967
- PMCID: PMC3295549
- DOI: 10.1200/JCO.2010.34.4010
Longer-term outcomes of letrozole versus placebo after 5 years of tamoxifen in the NCIC CTG MA.17 trial: analyses adjusting for treatment crossover
Abstract
Purpose: The interim analysis of the National Cancer Institute of Canada Clinical Trials Group MA.17 trial showed that letrozole was significantly better than placebo in disease-free survival (DFS) for postmenopausal women with hormone receptor-positive breast cancer following about 5 years of tamoxifen therapy. When patients were unblinded, those on placebo were offered letrozole. Longer-term efficacy of letrozole, especially survival, was of particular interest because the median follow-up of the first interim analysis was only 2.5 years. Efficacy was difficult to assess because more than 60% of placebo patients crossed over to letrozole after being unblinded.
Patients and methods: Two statistical approaches were used to adjust for the potential effects of treatment crossover: one was based on the inverse probability of censoring weighted (IPCW) Cox model and the other on a Cox model with time-dependent covariates.
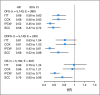
Results: With a median follow-up of 64 months, the hazard ratios (HRs) of letrozole and placebo from the IPCW analyses were HR of 0.52 (95% CI, 0.45 to 0.61; P < .001) for DFS, HR of 0.51 (95% CI, 0.42 to 0.61; P < .001) for distant disease-free survival (DDFS), and HR of 0.61 (95% CI, 0.52 to 0.71; P < .001) for overall survival (OS). The results from the analyses based on the Cox model with time-dependent covariates were similar for letrozole and placebo: HR of 0.58 (95% CI, 0.47 to 0.72; P < .001) for DFS, HR of 0.68 (95% CI, 0.52 to 0.88; P = .004) for DDFS, and HR of 0.76 (95% CI, 0.60 to 0.96; P = .02) for OS.
Conclusion: Exploratory analyses based on longer follow-up and adjusting for treatment crossover suggest that extended adjuvant letrozole was superior to placebo in DFS, DDFS, and OS.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures
Comment in
-
Deep time: the long and the short of adjuvant endocrine therapy for breast cancer.J Clin Oncol. 2012 Mar 1;30(7):684-6. doi: 10.1200/JCO.2011.40.1455. Epub 2012 Jan 23. J Clin Oncol. 2012. PMID: 22271478 No abstract available.
References
-
- Goss PE, Ingle JN, Martino S, et al. A randomized trial of letrozole in postmenopausal women after five years of tamoxifen therapy for early-stage breast cancer. N Engl J Med. 2003;349:1793–1802. - PubMed
-
- Goss PE, Ingle JN, Martino S, et al. Randomized trial of letrozole following tamoxifen as extended adjuvant therapy in receptor-positive breast cancer: Updated findings from NCIC CTG MA.17. J Natl Cancer Inst. 2005;97:1262–1271. - PubMed
-
- Ingle JN, Tu D, Pater JL, et al. Intent-to-treat analysis of the placebo-controlled trial of letrozole for extended adjuvant therapy in early breast cancer: NCIC CTG MA.17. Ann Oncol. 2008;19:877–882. - PubMed
-
- Goss PE, Ingle JN, Pater JL, et al. Late extended adjuvant treatment with letrozole improves outcome in women with early-stage breast cancer who complete 5 years of tamoxifen. J Clin Oncol. 2008;26:1948–1955. - PubMed
-
- Sommer A, Zeger SL. On estimating efficacy from clinical trials. Stat Med. 1991;10:45–52. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

