Treating obstructive sleep apnea with hypoglossal nerve stimulation
- PMID: 22043118
- PMCID: PMC3198202
- DOI: 10.5665/sleep.1380
Treating obstructive sleep apnea with hypoglossal nerve stimulation
Abstract
Background: Reduced upper airway muscle activity during sleep is fundamental to obstructive sleep apnea (OSA) pathogenesis. Hypoglossal nerve stimulation (HGNS) counteracts this problem, with potential to reduce OSA severity.
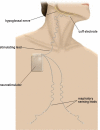
Study objectives: To examine safety and efficacy of a novel HGNS system (HGNS, Apnex Medical, Inc.) in treating OSA.
Participants: Twenty-one patients, 67% male, age (mean ± SD) 53.6 ± 9.2 years, with moderate to severe OSA and unable to tolerate continuous positive airway pressure (CPAP).
Design: Each participant underwent surgical implantation of the HGNS system in a prospective single-arm interventional trial. OSA severity was defined by apnea-hypopnea index (AHI) during in-laboratory polysomnography (PSG) at baseline and 3 and 6 months post-implant. Therapy compliance was assessed by nightly hours of use. Symptoms were assessed using the Epworth Sleepiness Scale (ESS), Functional Outcomes of Sleep Questionnaire (FOSQ), Calgary Sleep Apnea Quality of Life Index (SAQLI), and the Beck Depression Inventory (BDI).
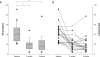
Results: HGNS was used on 89% ± 15% of nights (n = 21). On these nights, it was used for 5.8 ± 1.6 h per night. Nineteen of 21 participants had baseline and 6-month PSGs. There was a significant improvement (all P < 0.05) from baseline to 6 months in: AHI (43.1 ± 17.5 to 19.5 ± 16.7), ESS (12.1 ± 4.7 to 8.1 ± 4.4), FOSQ (14.4 ± 2.0 to 16.7 ± 2.2), SAQLI (3.2 ± 1.0 to 4.9 ± 1.3), and BDI (15.8 ± 9.0 to 9.7 ± 7.6). Two serious device-related adverse events occurred: an infection requiring device removal and a stimulation lead cuff dislodgement requiring replacement.
Conclusions: HGNS demonstrated favorable safety, efficacy, and compliance. Participants experienced a significant decrease in OSA severity and OSA-associated symptoms.
Clinical trial information: NAME: Australian Clinical Study of the Apnex Medical HGNS System to Treat Obstructive Sleep Apnea.
Registration number: NCT01186926. URL: http://clinicaltrials.gov/ct2/show/NCT01186926.
Keywords: Sleep apnea; genioglossus muscle; hypoglossal nerve stimulation; implantable neurostimulator; lung.
Figures


Comment in
-
Back to the future or forward to the past?Sleep. 2011 Nov 1;34(11):1455-6. doi: 10.5665/sleep.1370. Sleep. 2011. PMID: 22043113 Free PMC article. No abstract available.
References
-
- Remmers JE, deGroot WJ, Sauerland EK, Anch AM. Pathogenesis of upper airway occlusion during sleep. J Appl Physiol. 1978;44:931–8. - PubMed
-
- Young T, Evans L, Finn L, Palta M. Estimation of the clinically diagnosed proportion of sleep apnea syndrome in middle-aged men and women. Sleep. 1997;20:705–6. - PubMed
-
- Gay P, Weaver T, Loube D, Iber C. Evaluation of positive airway pressure treatment for sleep related breathing disorders in adults. Sleep. 2006;29:381–401. - PubMed
-
- Kribbs NB, Pack AI, Kline LR, et al. Objective measurement of patterns of nasal CPAP use by patients with obstructive sleep apnea. Am Rev Respir Dis. 1993;147:887–95. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

