The atypical stimulant and nootropic modafinil interacts with the dopamine transporter in a different manner than classical cocaine-like inhibitors
- PMID: 22043293
- PMCID: PMC3197159
- DOI: 10.1371/journal.pone.0025790
The atypical stimulant and nootropic modafinil interacts with the dopamine transporter in a different manner than classical cocaine-like inhibitors
Erratum in
- PLoS One. 2012;7(1): doi.10.1371/annotation/ba711a7c-13fb-4d18-89a8-28dcc68fcd04.
Abstract
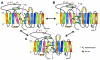
Modafinil is a mild psychostimulant with pro-cognitive and antidepressant effects. Unlike many conventional stimulants, modafinil has little appreciable potential for abuse, making it a promising therapeutic agent for cocaine addiction. The chief molecular target of modafinil is the dopamine transporter (DAT); however, the mechanistic details underlying modafinil's unique effects remain unknown. Recent studies suggest that the conformational effects of a given DAT ligand influence the magnitude of the ligand's reinforcing properties. For example, the atypical DAT inhibitors benztropine and GBR12909 do not share cocaine's notorious addictive liability, despite having greater binding affinity. Here, we show that the binding mechanism of modafinil is different than cocaine and similar to other atypical inhibitors. We previously established two mutations (W84L and D313N) that increase the likelihood that the DAT will adopt an outward-facing conformational state--these mutations increase the affinity of cocaine-like inhibitors considerably, but have little or opposite effect on atypical inhibitor binding. Thus, a compound's WT/mutant affinity ratio can indicate whether the compound preferentially interacts with a more outward- or inward-facing conformational state. Modafinil displayed affinity ratios similar to those of benztropine, GBR12909 and bupropion (which lack cocaine-like effects in humans), but far different than those of cocaine, β-CFT or methylphenidate. Whereas treatment with zinc (known to stabilize an outward-facing transporter state) increased the affinity of cocaine and methylphenidate two-fold, it had little or no effect on the binding of modafinil, benztropine, bupropion or GBR12909. Additionally, computational modeling of inhibitor binding indicated that while β-CFT and methylphenidate stabilize an "open-to-out" conformation, binding of either modafinil or bupropion gives rise to a more closed conformation. Our findings highlight a mechanistic difference between modafinil and cocaine-like stimulants and further demonstrate that the conformational effects of a given DAT inhibitor influence its phenomenological effects.
Conflict of interest statement
Figures
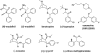

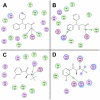
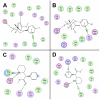
 +) or aromatic π-stacking (
+) or aromatic π-stacking (
 ). (A, B) Residue interaction maps for modafinil bound at the S1 (A) and S2 sites (B). (C, D) Interaction maps for bupropion bound at the S1 (C) and S2 sites (D), respectively. For both of the atypical inhibitors, binding at the S1 site (panels A and C) gives rise to few strong interactions with the DAT—only their protonated nitrogen atoms form hydrogen bonds—suggesting that recognition of these relatively modest inhibitors (K
i>100 nM) is influenced more by molecular shape and steric bulk than by specific polar interactions.
). (A, B) Residue interaction maps for modafinil bound at the S1 (A) and S2 sites (B). (C, D) Interaction maps for bupropion bound at the S1 (C) and S2 sites (D), respectively. For both of the atypical inhibitors, binding at the S1 site (panels A and C) gives rise to few strong interactions with the DAT—only their protonated nitrogen atoms form hydrogen bonds—suggesting that recognition of these relatively modest inhibitors (K
i>100 nM) is influenced more by molecular shape and steric bulk than by specific polar interactions.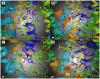
References
-
- Turner DC, Clark L, Dowson J, Robbins TW, Sahakian BJ. Modafinil improves cognition and response inhibition in adult attention-deficit/hyperactivity disorder. Biol Psychiatry. 2004;55:1031–1040. - PubMed
-
- Marchant N, Kamel F, Echlin K, Grice J, Lewis M, et al. Modafinil improves rapid shifts of attention. Psychopharmacology. 2009;202:487–495. - PubMed
-
- Tsanov M, Lyons DG, Barlow S, González Reyes RE, O'Mara SM. The psychostimulant modafinil facilitates water maze performance and augments synaptic potentiation in dentate gyrus. Neuropharmacology. 2010;59:9–19. - PubMed
-
- Regenthal R, Koch H, Köhler C, Preiss R, Krügel U. Depression-like deficits in rats improved by subchronic modafinil. Psychopharmacology. 2009;204:627–639. - PubMed
-
- Ninan PT, Hassman HA, Glass SJ, McManus FC. Adjunctive modafinil at initiation of treatment with a selective serotonin reuptake inhibitor enhances the degree and onset of therapeutic effects in patients with major depressive disorder and fatigue. J Clinical Psychiatry. 2004;65:414–420. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

