Migfilin and filamin as regulators of integrin activation in endothelial cells and neutrophils
- PMID: 22043318
- PMCID: PMC3197140
- DOI: 10.1371/journal.pone.0026355
Migfilin and filamin as regulators of integrin activation in endothelial cells and neutrophils
Abstract
Cell adhesion and migration depend on engagement of extracellular matrix ligands by integrins. Integrin activation is dynamically regulated by interactions of various cytoplasmic proteins, such as filamin and integrin activators, talin and kindlin, with the cytoplasmic tail of the integrin β subunit. Although filamin has been suggested to be an inhibitor of integrin activation, direct functional evidence for the inhibitory role of filamin is limited. Migfilin, a filamin-binding protein enriched at cell-cell and cell-extracellular matrix contact sites, can displace filamin from β1 and β3 integrins and promote integrin activation. However, its role in activation and functions of different β integrins in human vascular cells is unknown. In this study, using flow cytometry, we demonstrate that filamin inhibits β1 and αIIbβ3 integrin activation, and migfilin can overcome its inhibitory effect. Migfilin protein is widely expressed in different adherent and circulating blood cells and can regulate integrin activation in naturally-occurring vascular cells, endothelial cells and neutrophils. Migfilin can activate β1, β2 and β3 integrins and promote integrin mediated responses while migfilin depletion impairs the spreading and migration of endothelial cells. Thus, filamin can act broadly as an inhibitor and migfilin is a promoter of integrin activation.
Conflict of interest statement
Figures




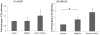
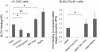
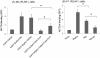
Similar articles
-
Structural basis of the migfilin-filamin interaction and competition with integrin beta tails.J Biol Chem. 2008 Dec 12;283(50):35154-63. doi: 10.1074/jbc.M802592200. Epub 2008 Sep 30. J Biol Chem. 2008. PMID: 18829455 Free PMC article.
-
Migfilin, a molecular switch in regulation of integrin activation.J Biol Chem. 2009 Feb 13;284(7):4713-22. doi: 10.1074/jbc.M807719200. Epub 2008 Dec 13. J Biol Chem. 2009. PMID: 19074766 Free PMC article.
-
Evidence for multisite ligand binding and stretching of filamin by integrin and migfilin.Biochemistry. 2011 May 24;50(20):4229-31. doi: 10.1021/bi2003229. Epub 2011 Apr 27. Biochemistry. 2011. PMID: 21524097 Free PMC article.
-
Filamin A mediates interactions between cytoskeletal proteins that control cell adhesion.FEBS Lett. 2011 Jan 3;585(1):18-22. doi: 10.1016/j.febslet.2010.11.033. Epub 2010 Nov 21. FEBS Lett. 2011. PMID: 21095189 Review.
-
Regulation of integrin activation.Annu Rev Cell Dev Biol. 2011;27:321-45. doi: 10.1146/annurev-cellbio-100109-104104. Epub 2011 Jun 10. Annu Rev Cell Dev Biol. 2011. PMID: 21663444 Review.
Cited by
-
Structural mechanism of integrin inactivation by filamin.Nat Struct Mol Biol. 2015 May;22(5):383-9. doi: 10.1038/nsmb.2999. Epub 2015 Apr 6. Nat Struct Mol Biol. 2015. PMID: 25849143 Free PMC article.
-
The focal adhesion protein kindlin-2 controls mitotic spindle assembly by inhibiting histone deacetylase 6 and maintaining α-tubulin acetylation.J Biol Chem. 2020 May 1;295(18):5928-5943. doi: 10.1074/jbc.RA120.012954. Epub 2020 Mar 13. J Biol Chem. 2020. PMID: 32169902 Free PMC article.
-
Moving towards a paradigm: common mechanisms of chemotactic signaling in Dictyostelium and mammalian leukocytes.Cell Mol Life Sci. 2014 Oct;71(19):3711-47. doi: 10.1007/s00018-014-1638-8. Epub 2014 May 21. Cell Mol Life Sci. 2014. PMID: 24846395 Free PMC article. Review.
-
Regulation of actin cytoskeletal dynamics in T cell development and function.Front Immunol. 2025 Jun 10;16:1622928. doi: 10.3389/fimmu.2025.1622928. eCollection 2025. Front Immunol. 2025. PMID: 40557146 Free PMC article. Review.
-
NMR Structure, Dynamics and Interactions of the Integrin β2 Cytoplasmic Tail with Filamin Domain IgFLNa21.Sci Rep. 2018 Apr 3;8(1):5490. doi: 10.1038/s41598-018-23866-6. Sci Rep. 2018. PMID: 29615775 Free PMC article.
References
-
- Kiema T, Lad Y, Jiang P, Oxley CL, Baldassarre M, et al. The molecular basis of filamin binding to integrins and competition with talin. Mol Cell. 2006;21:337–347. - PubMed
-
- Takala H, Nurminen E, Nurmi SM, Aatonen M, Strandin T, et al. Beta2 integrin phosphorylation on Thr758 acts as a molecular switch to regulate 14-3-3 and filamin binding. Blood. 2008;112:1853–1862. blood-2007-12-127795 [pii]; doi: 10.1182/blood-2007-12-127795. - DOI - PubMed
-
- Ithychanda SS, Das M, Ma YQ, Ding K, Wang X, et al. Migfilin, a molecular switch in regulation of integrin activation. J Biol Chem. 2009;284:4713–4722. M807719200 [pii]; doi: 10.1074/jbc.M807719200. - DOI - PMC - PubMed
-
- Ithychanda SS, Hsu D, Li H, Yan L, Liu DD, et al. Identification and characterization of multiple similar ligand-binding repeats in filamin: implication on filamin-mediated receptor clustering and cross-talk. J Biol Chem. 2009;284:35113–35121. M109.060954 [pii];doi: 10.1074/jbc.M109.060954. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

