UV-sensitive photoreceptor protein OPN5 in humans and mice
- PMID: 22043319
- PMCID: PMC3197025
- DOI: 10.1371/journal.pone.0026388
UV-sensitive photoreceptor protein OPN5 in humans and mice
Abstract
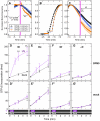
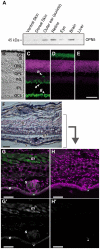
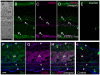
A variety of animal species utilize the ultraviolet (UV) component of sunlight as their environmental cues, whereas physiological roles of UV photoreception in mammals, especially in human beings, remain open questions. Here we report that mouse neuropsin (OPN5) encoded by the Opn5 gene exhibited an absorption maximum (λmax) at 380 nm when reconstituted with 11-cis-retinal. Upon UV-light illumination, OPN5 was converted to a blue-absorbing photoproduct (λmax 470 nm), which was stable in the dark and reverted to the UV-absorbing state by the subsequent orange light illumination, indicating its bistable nature. Human OPN5 also had an absorption maximum at 380 nm with spectral properties similar to mouse OPN5, revealing that OPN5 is the first and hitherto unknown human opsin with peak sensitivity in the UV region. OPN5 was capable of activating heterotrimeric G protein Gi in a UV-dependent manner. Immuno-blotting analyses of mouse tissue extracts identified the retina, the brain and, unexpectedly, the outer ears as the major sites of OPN5 expression. In the tissue sections of mice, OPN5 immuno-reactivities were detected in a subset of non-rod/non-cone retinal neurons as well as in the epidermal and muscle cells of the outer ears. Most of these OPN5-immuno-reactivities in mice were co-localized with positive signals for the alpha-subunit of Gi. These results demonstrate the first example of UV photoreceptor in human beings and strongly suggest that OPN5 triggers a UV-sensitive Gi-mediated signaling pathway in the mammalian tissues.
Conflict of interest statement
Figures


References
-
- Eisner T, Silberglied RE, Aneshansley D, Carrel JE, Howland HC. Ultraviolet video-viewing: the television camera as an insect eye. Science. 1969;166:1172–4. - PubMed
-
- Froy O, Gotter AL, Casselman AL, Reppert SM. Illuminating the circadian clock in monarch butterfly migration. Science. 2003;300:1303–5. - PubMed
-
- Bennett A, Cuthill I, Partridge J, Maier E. Ultraviolet vision and mate choice in zebra finches. Nature. 1996;380:433–5.
-
- Jourdie V, Moureau B, Bennett A, Heeb P. Ecology: ultraviolet reflectance by the skin of nestlings. Nature. 2004;431:262. - PubMed
-
- Jacobs GH. Ultraviolet vision in vertebrates. Amer Zool. 1992;32:544–554.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

