Role of the inflammatory protein serine protease inhibitor Kazal in preventing cytolytic granule granzyme A-mediated apoptosis
- PMID: 22043941
- PMCID: PMC3230794
- DOI: 10.1111/j.1365-2567.2011.03498.x
Role of the inflammatory protein serine protease inhibitor Kazal in preventing cytolytic granule granzyme A-mediated apoptosis
Abstract
Serine protease inhibitor Kazal (SPIK) is an inflammatory protein whose levels are elevated in numerous cancers. However, the role of this protein in cancer development is unknown. We have recently found that SPIK suppresses serine protease-dependent cell apoptosis. Here, we report that anti-SPIK antibodies can co-immmunoprecipitate serine protease granzyme A (GzmA), a cytolytic granule secreted by cytotoxic T lymphocytes and natural killer cells during immune surveillance, and that SPIK suppresses GzmA-induced cell apoptosis. Deletion studies show that the C3-C4 region of SPIK is critical for this suppression. These studies suggest that over-expression of SPIK may prevent GzmA-mediated immune-killing, thereby establishing the tolerance of cancer cells to the body's immune surveillance system. Suppression of over-expressed SPIK can restore the susceptibility of these cells to apoptotic death triggered by GzmA. This finding implies that it is possible to overcome tolerance of cancer cells to the body's immune surveillance system and restore the GzmA-mediated immune-killing by suppressing the over-expression of SPIK.
© 2011 The Authors. Immunology © 2011 Blackwell Publishing Ltd.
Figures


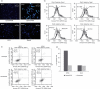


Similar articles
-
Tumor-associated protein SPIK/TATI suppresses serine protease dependent cell apoptosis.Apoptosis. 2008 Apr;13(4):483-94. doi: 10.1007/s10495-008-0193-x. Apoptosis. 2008. PMID: 18347987
-
Granzymes in cancer and immunity.Cell Death Differ. 2010 Apr;17(4):616-23. doi: 10.1038/cdd.2009.206. Epub 2010 Jan 15. Cell Death Differ. 2010. PMID: 20075940 Review.
-
Expression of the granzyme B inhibitor, protease inhibitor 9, by tumor cells in patients with non-Hodgkin and Hodgkin lymphoma: a novel protective mechanism for tumor cells to circumvent the immune system?Blood. 2002 Jan 1;99(1):232-7. doi: 10.1182/blood.v99.1.232. Blood. 2002. PMID: 11756176
-
Target cell apoptosis induced by cytotoxic T cells and natural killer cells involves synergy between the pore-forming protein, perforin, and the serine protease, granzyme B.Aust N Z J Med. 1995 Dec;25(6):793-9. doi: 10.1111/j.1445-5994.1995.tb02883.x. Aust N Z J Med. 1995. PMID: 8770355 Review.
-
Proapoptotic functions of cytotoxic lymphocyte granule constituents in vitro and in vivo.Curr Opin Immunol. 2000 Jun;12(3):323-9. doi: 10.1016/s0952-7915(00)00094-7. Curr Opin Immunol. 2000. PMID: 10781403 Review.
Cited by
-
Structural and Biophysical Insights into SPINK1 Bound to Human Cationic Trypsin.Int J Mol Sci. 2022 Mar 23;23(7):3468. doi: 10.3390/ijms23073468. Int J Mol Sci. 2022. PMID: 35408828 Free PMC article.
-
SPINK1 promotes colorectal cancer progression by downregulating Metallothioneins expression.Oncogenesis. 2015 Aug 10;4(8):e162. doi: 10.1038/oncsis.2015.23. Oncogenesis. 2015. PMID: 26258891 Free PMC article.
-
Circulating Exosomes Are Strongly Involved in SARS-CoV-2 Infection.Front Mol Biosci. 2021 Feb 22;8:632290. doi: 10.3389/fmolb.2021.632290. eCollection 2021. Front Mol Biosci. 2021. PMID: 33693030 Free PMC article.
-
Liver Cancer-Specific Serine Protease Inhibitor Kazal Is a Potentially Novel Biomarker for the Early Detection of Hepatocellular Carcinoma.Clin Transl Gastroenterol. 2020 Dec;11(12):e00271. doi: 10.14309/ctg.0000000000000271. Clin Transl Gastroenterol. 2020. PMID: 33512798 Free PMC article.
-
SPINK1 Promoter Variants Are Associated with Prostate Cancer Predisposing Alterations in Benign Prostatic Hyperplasia Patients.Anticancer Res. 2015 Jul;35(7):3811-9. Anticancer Res. 2015. PMID: 26124326 Free PMC article.
References
-
- Stenman UH. Tumor-associated trypsin inhibitor. Clin Chem. 2002;48:1206–9. - PubMed
-
- Greene LJ. Pancreatic exocrine secretory proteins. J Surg Oncol. 1975;7:151–4. - PubMed
-
- Tomita N, Doi S, Higashiyama M, et al. Expression of pancreatic secretory trypsin inhibitor gene in human colorectal tumor. Cancer. 1990;66:2144–9. - PubMed
-
- Ohmachi Y, Murata A, Matsuura N, et al. Specific expression of the pancreatic-secretory-trypsin-inhibitor (PSTI) gene in hepatocellular carcinoma. Int J Cancer. 1993;55:728–34. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

