miR-155 modulates microglia-mediated immune response by down-regulating SOCS-1 and promoting cytokine and nitric oxide production
- PMID: 22043967
- PMCID: PMC3246654
- DOI: 10.1111/j.1365-2567.2011.03514.x
miR-155 modulates microglia-mediated immune response by down-regulating SOCS-1 and promoting cytokine and nitric oxide production
Abstract
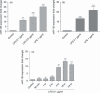
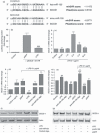
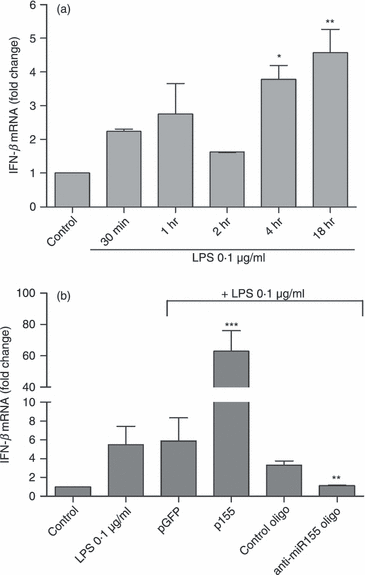
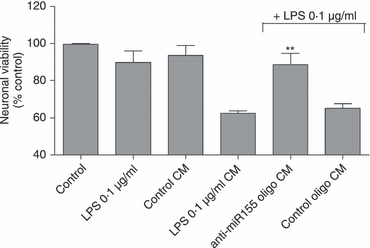
Innate immunity constitutes the first line of defence against both external and endogenous threats in the brain, and microglia cells are considered key mediators of this process. Recent studies have shown that microRNAs (miRNAs) may play a determinant role in the regulation of gene expression during innate immune responses. The major goal of this work was to investigate the contribution of a specific miRNA - miR-155 - to the modulation of the microglia-mediated immune response. For this purpose, in vitro studies were performed in N9 microglia cells to evaluate changes in the levels of this miRNA following microglia activation. A strong up-regulation of miR-155 expression was observed following microglia exposure to lipopolysaccharide, which was consistent with a decrease in the levels of the suppressor of cytokine signalling 1 (SOCS-1) protein, a key inhibitor of the inflammatory process and a predicted target of miR-155. The miR-155 knockdown by anti-miRNA oligonucleotides up-regulated SOCS-1 mRNA and protein levels and significantly decreased the production of nitric oxide and the expression of inflammatory cytokines and inducible nitric oxide synthase. Finally, treatment of neuronal primary cultures with conditioned medium obtained from microglia cells, in which miR-155 was inhibited before cell activation, decreased inflammatory-mediated neuronal cell death. Overall, our results show that miR-155 has a pro-inflammatory role in microglia and is necessary for the progression of the immune response through the modulation of SOCS-1, suggesting that, in a chronic inflammatory context, miR-155 inhibition can have a neuroprotective effect.
© 2011 The Authors. Immunology © 2011 Blackwell Publishing Ltd.
Figures

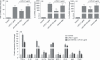

Similar articles
-
Highly purified lipoteichoic acid induced pro-inflammatory signalling in primary culture of rat microglia through Toll-like receptor 2: selective potentiation of nitric oxide production by muramyl dipeptide.J Neurochem. 2006 Oct;99(2):596-607. doi: 10.1111/j.1471-4159.2006.04085.x. Epub 2006 Jul 31. J Neurochem. 2006. PMID: 16879708
-
microRNA-200b modulates microglia-mediated neuroinflammation via the cJun/MAPK pathway.J Neurochem. 2014 Aug;130(3):388-401. doi: 10.1111/jnc.12731. Epub 2014 May 9. J Neurochem. 2014. PMID: 24749688
-
Involvement of suppressor of cytokine signaling-3 as a mediator of the inhibitory effects of IL-10 on lipopolysaccharide-induced macrophage activation.J Immunol. 2002 Jun 15;168(12):6404-11. doi: 10.4049/jimmunol.168.12.6404. J Immunol. 2002. PMID: 12055259
-
Understanding the role of SOCS signaling in neurodegenerative diseases: Current and emerging concepts.Cytokine Growth Factor Rev. 2017 Oct;37:67-79. doi: 10.1016/j.cytogfr.2017.07.005. Epub 2017 Aug 1. Cytokine Growth Factor Rev. 2017. PMID: 28801024 Review.
-
Involvement of microRNA in microglia-mediated immune response.Clin Dev Immunol. 2013;2013:186872. doi: 10.1155/2013/186872. Epub 2013 May 23. Clin Dev Immunol. 2013. PMID: 23762086 Free PMC article. Review.
Cited by
-
Chronic morphine-induced microRNA-124 promotes microglial immunosuppression by modulating P65 and TRAF6.J Immunol. 2015 Feb 1;194(3):1021-30. doi: 10.4049/jimmunol.1400106. Epub 2014 Dec 24. J Immunol. 2015. PMID: 25539811 Free PMC article.
-
Epigenetic Regulation of Neuroinflammation in Parkinson's Disease.Int J Mol Sci. 2021 May 7;22(9):4956. doi: 10.3390/ijms22094956. Int J Mol Sci. 2021. PMID: 34066949 Free PMC article. Review.
-
Astrocytic MicroRNA in Ageing, Inflammation, and Neurodegenerative Disease.Front Physiol. 2022 Feb 10;12:826697. doi: 10.3389/fphys.2021.826697. eCollection 2021. Front Physiol. 2022. PMID: 35222067 Free PMC article. Review.
-
MicroRNAs in Microglia: How do MicroRNAs Affect Activation, Inflammation, Polarization of Microglia and Mediate the Interaction Between Microglia and Glioma?Front Mol Neurosci. 2019 May 10;12:125. doi: 10.3389/fnmol.2019.00125. eCollection 2019. Front Mol Neurosci. 2019. PMID: 31133802 Free PMC article. Review.
-
Epigenetics and the modulation of neuroinflammation.Neurotherapeutics. 2013 Oct;10(4):782-8. doi: 10.1007/s13311-013-0207-4. Neurotherapeutics. 2013. PMID: 23963788 Free PMC article. Review.
References
-
- Czlonkowska A, Kurkowska-Jastrzebska I. Inflammation and gliosis in neurological diseases – clinical implications. J Neuroimmunol. 2010;231:78–85. - PubMed
-
- Lehnardt S. Innate immunity and neuroinflammation in the CNS: the role of microglia in Toll-like receptor-mediated neuronal injury. Glia. 2010;58:253–63. - PubMed
-
- Qin L, Liu Y, Cooper C, Liu B, Wilson B, Hong JS. Microglia enhance β-amyloid peptide-induced toxicity in cortical and mesencephalic neurons by producing reactive oxygen species. J Neurochem. 2002;83:973–83. - PubMed
-
- Takeuchi H, Wang J, Kawanokuchi J, Mitsuma N, Mizuno T, Suzumura A. Interferon-gamma induces microglial-activation-induced cell death: a hypothetical mechanism of relapse and remission in multiple sclerosis. Neurobiol Dis. 2006;22:33–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

