Diversity of Chlamydomonas channelrhodopsins
- PMID: 22044280
- PMCID: PMC3253254
- DOI: 10.1111/j.1751-1097.2011.01027.x
Diversity of Chlamydomonas channelrhodopsins
Abstract
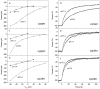
Channelrhodopsins act as photoreceptors for control of motility behavior in flagellates and are widely used as genetically targeted tools to optically manipulate the membrane potential of specific cell populations ("optogenetics"). The first two channelrhodopsins were obtained from the model organism Chlamydomonas reinhardtii (CrChR1 and CrChR2). By homology cloning we identified three new channelrhodopsin sequences from the same genus, CaChR1, CyChR1 and CraChR2, from C. augustae, C. yellowstonensis and C. raudensis, respectively. CaChR1 and CyChR1 were functionally expressed in HEK293 cells, where they acted as light-gated ion channels similar to CrChR1. However, both, which are similar to each other, differed from CrChR1 in current kinetics, inactivation, light intensity dependence, spectral sensitivity and dependence on the external pH. These results show that extensive channelrhodopsin diversity exists even within the same genus, Chlamydomonas. The maximal spectral sensitivity of CaChR1 was at 520 nm at pH 7.4, about 40 nm redshifted as compared to that of CrChR1 under the same conditions. CaChR1 was successfully expressed in Pichia pastoris and exhibited an absorption spectrum identical to the action spectrum of CaChR1-generated photocurrents. The redshifted spectra and the lack of fast inactivation in CaChR1- and CyChR1-generated currents are features desirable for optogenetics applications.
© 2011 Wiley Periodicals, Inc. Photochemistry and Photobiology © 2011 The American Society of Photobiology.
Figures





References
-
- Foster KW, Saranak J, Patel N, Zarrilli G, Okabe M, Kline T, Nakanishi K. A rhodopsin is the functional photoreceptor for phototaxis in the unicelullar eukaryote Chlamydomonas. Nature. 1984;311:756–759. - PubMed
-
- Asamizu E, Miura K, Kucho K, Inoue Y, Fukuzawa H, Ohyama K, Nakamura Y, Tabata S. Generation of expressed sequence tags from low-CO2 and high-CO2 adapted cells of Chlamydomonas reinhardtii. DNA Research. 2000;7:305–307. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

